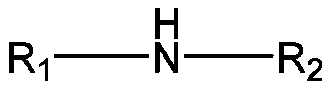Preparation method of thiadiazole derivative
A technology for thiadiazole derivatives and substances, which is applied in the field of preparation of thiadiazole derivatives, can solve the problems of safety risks in the process, difficult separation, low sulfur content in products, etc., and achieves good copper corrosion inhibition effect and easy purification of products. , good oil solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
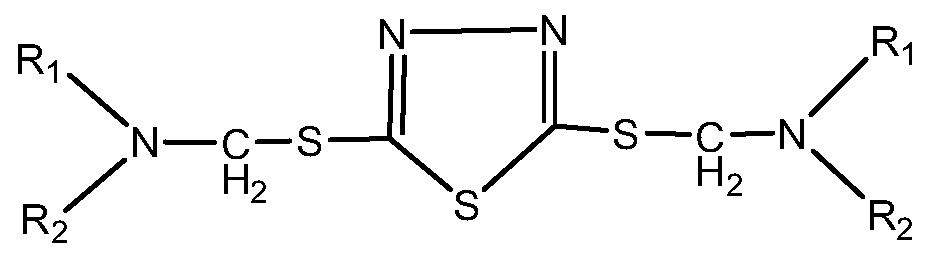
[0030] The invention provides a preparation method of thiadiazole derivatives, using the principle of Mannich reaction, using aliphatic amines such as alkyl primary amines or secondary amines, formaldehyde aqueous solution or paraformaldehyde, and DMTD as raw materials, through one-step synthesis Thiadiazole derivatives.
[0031] The preparation method of the present invention comprises:
[0032] Add fatty amines dropwise to the formaldehyde solution of DMTD. After the dropwise addition, react at 60-100°C for 2-5 hours, which is the first reaction stage; then increase the reaction temperature by 10-50°C and react again for 2-5 hours 5h, this is the second reaction stage; after the reaction is completed, distill under reduced pressure and filter to obtain thiadiazole derivatives.
[0033] The preparation method of the present invention specifically comprises:
[0034] Step 1. Add DMTD and formaldehyde or DMTD, formaldehyde and solvent into the reaction vessel, and use any sti...
Embodiment 1
[0051] The invention provides a preparation method of thiadiazole derivatives, comprising:
[0052] In a clean three-necked flask with a stirring and heating device, add 10g of dimercaptothiadiazole, 10g of ethanol and 4.0g of 30% formaldehyde, and stir thoroughly; raise the temperature to 65°C, add 26.0g of dodecylamine dropwise, and add After completion, keep the reaction for 2 hours; then raise the temperature to 80°C and continue the reaction for 4 hours. Distill under reduced pressure (operating conditions are -0.095Mpa, 100°C, 1.5h), and obtain the finished product after filtration. The active ingredient content of the finished product is 99.3% (calculated by sulfur content, the test method for the active ingredient content of the finished product is attached).
[0053] Attachment: Test method for effective component content of finished product
[0054] Detection of sulfur content S in the product 1 , the theoretical sulfur content S calculated according to the molecu...
Embodiment 2
[0057] The invention provides a preparation method of thiadiazole derivatives, comprising:
[0058] In a clean three-necked flask with a stirring and heating device, add 10g of dimercaptothiadiazole, 10g of water and 6.0g of 30% formaldehyde, stir well; raise the temperature to 70°C, add 33g of tridecylamine drop by drop, and the dropwise addition is completed Then keep warm for 3h; then raise the temperature to 100°C and continue the reaction for 2h; distill under reduced pressure (operating conditions are -0.095Mpa, 100°C, 1.5h), and obtain the finished product after filtration. The content of active components in the finished product is 99.1% (calculated by sulfur content).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com