A kind of phosphorus anion reagent and its preparation method and application
An anion and reagent technology, applied in the field of phosphorus anion reagents and their preparation, can solve the problems of harsh reaction conditions, large substrate limitations, and large energy consumption, and achieve the effects of loose reaction conditions, low reaction energy, and safe synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of bis(trichlorosilyl)phosphorus anion tetrabutylammonium salt:
[0035]
[0036] The specific operations are as follows:
[0037] In a glove box filled with inert gas Ar, weigh 339 mg of tetrabutylammonium dihydrogen phosphate (1 mmol) into an autoclave (pressure is 1 MPa), add 10 ml of trichlorosilane, heat to 120 ° C and stir for 48 h, the reaction The solution was washed with 10 ml of n-hexane and dried to obtain 1.2 g of white powder with a yield of 72%.
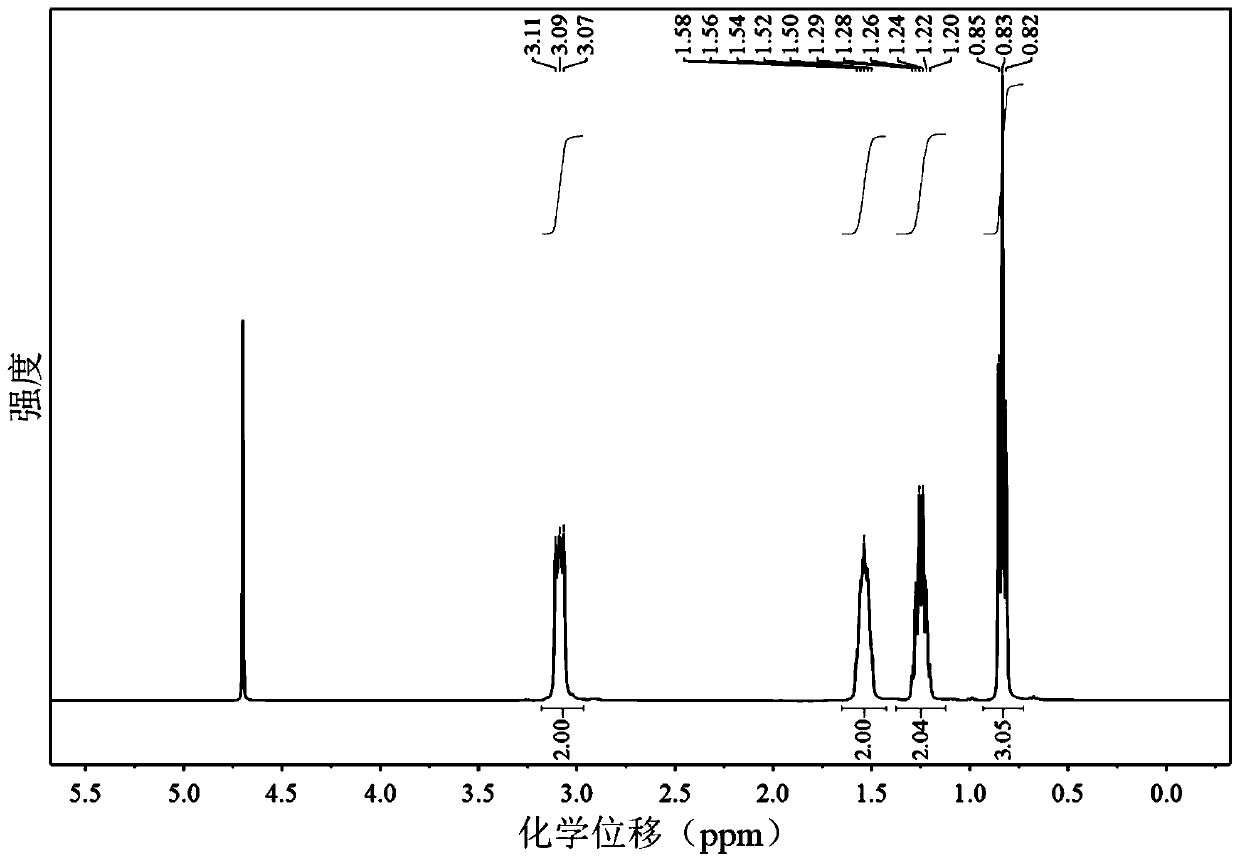
[0038] 1 H NMR (400MHz, D 2 O)δ3.27-2.89(m,2H),1.69-1.39(m,2H),1.39-1.12(m,2H),0.83(t,J=7.3Hz,3H).1.00(t,J=7.3 Hz, 3H).
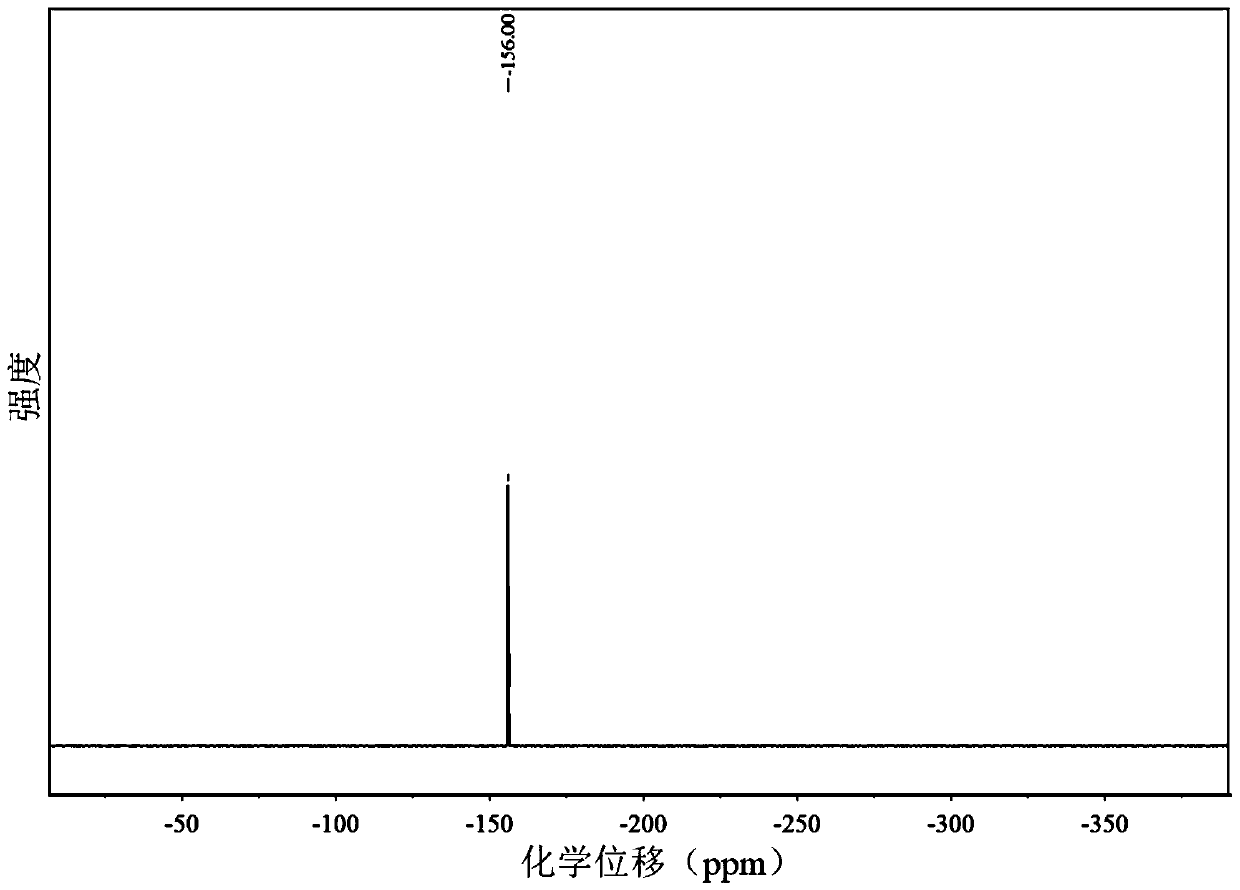
[0039] 31 P NMR (162MHz, CDCl 3 )δ-156.
Embodiment 2
[0041] Synthesis of bis(dichloroboryl)phosphorus anion tetrabutylammonium salt:
[0042]
[0043] The specific operations are as follows:
[0044] In a glove box filled with inert gas He, weigh 339 mg of tetrabutylammonium dihydrogen phosphite (1 mmol) into an autoclave (pressure is 1 MPa), add 10 ml of butyl dichloroborane, heat to 120°C and stir After 48 hours of reaction, the reaction solution was washed with 10 ml of n-hexane and then dried to obtain 1.4 g of white powder with a yield of 68%.
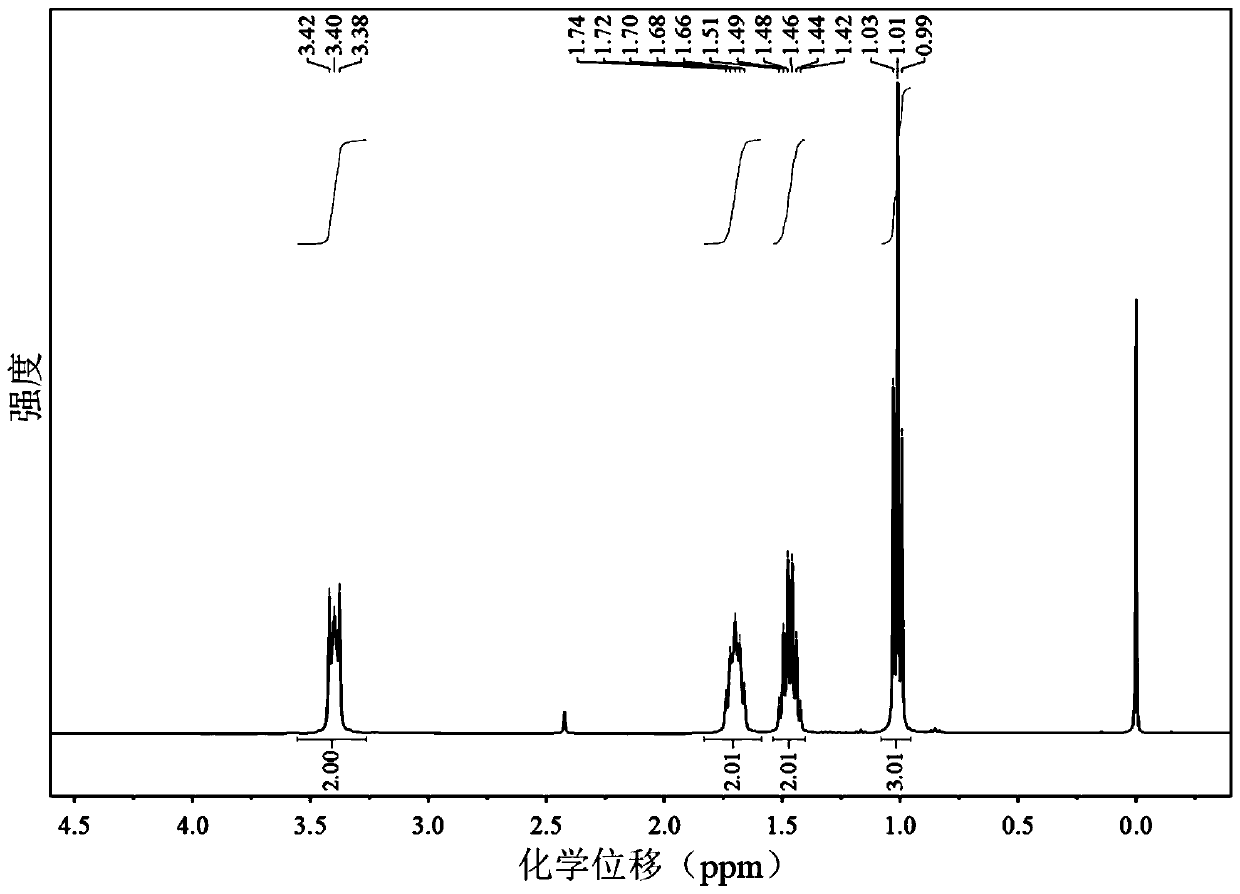
[0045] 1 H NMR (400MHz, CDCl 3 )δ3.52–3.29(m,2H),1.79–1.62(m,2H),1.55–1.39(m,2H),1.01(t,J=7.3Hz,3H).
Embodiment 3
[0047] Synthesis of bis(dichloroaluminum) phosphorus anion tetrabutylammonium salt:
[0048]
[0049] The specific operations are as follows:
[0050] In a glove box filled with inert gas Ne, weigh 339 mg of tetrabutylammonium dihydrogen hypophosphite (1 mmol) into an autoclave (pressure is 1 MPa), add 5 g of ethyl aluminum dichloride, and heat 10 ml of tetrahydrofuran to 120° C. The reaction was stirred for 48 h, the reaction solution was washed with 10 ml of n-hexane and then dried to obtain 1.05 g of yellow powder with a yield of 57%.
[0051] Elemental Analysis C 16 H 36 Cl 4 N 1 P 1 Al 2 : Calculated C 40.94, H 7.75, N 3.03, Theoretical C 40.96, H7.73, N 2.99.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com