A method of using black phosphorus to synthesize organic phosphine compounds
A compound and organic phosphine technology, which is applied in the field of synthesis of organic phosphine compounds using black phosphorus, can solve the problems of not meeting the development requirements of green chemistry, harsh reaction conditions, and large substrate limitations, and achieve safe and efficient synthesis with less reaction by-products , good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the synthetic method one of triethyl phosphate
[0046]
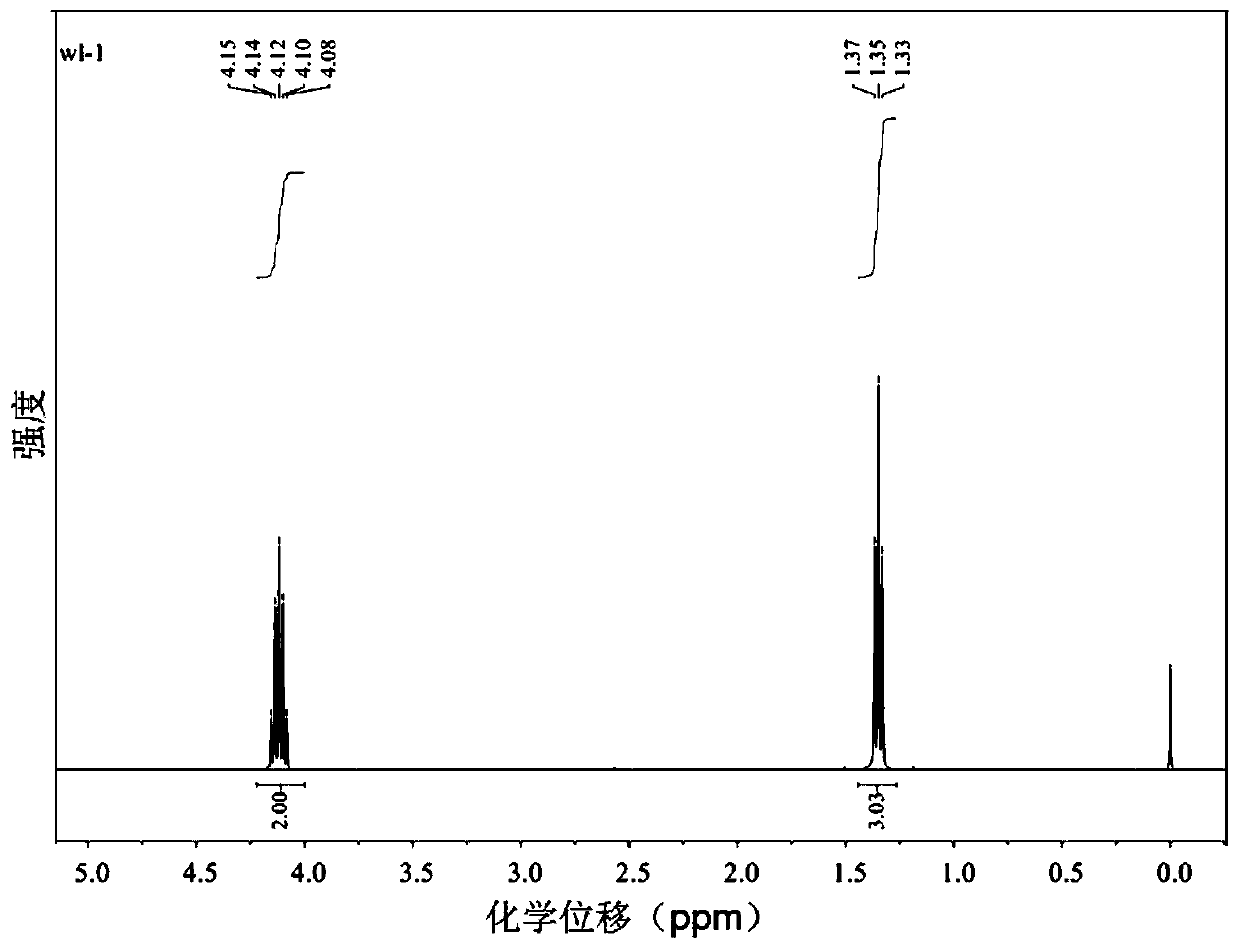
[0047] Take 6.2mg of black phosphorus crystals and disperse them in 20mL of ethanol, add 2mL of H 2 o 2 , stirred and reacted for 4h at 40°C, stopped the reaction after the solution was clarified, then removed the solvent by rotary evaporation, and obtained the yellow oily product triethyl phosphate after separation by silica gel column chromatography, and its yield was 75%, and its nuclear magnetic spectrum was determined as follows: figure 1 As shown, NMR spectrum data: 1 H NMR (400MHz, CDCl 3 )δ4.23–4.00(m,6H),1.35(m,9H), the spectrum proves the correctness of its structure.
Embodiment 2
[0048] Embodiment 2: the synthetic method two of triethyl phosphate
[0049]
[0050] Disperse 6.2mg of black phosphorus crystals in 20ml of ethanol, add 20mg of KClO to the system 3 , Stir the reaction at 60°C for 8h, stop the reaction after the solution is clear, then remove the solvent by rotary evaporation, and obtain the yellow oily product triethyl phosphate after separation by silica gel column chromatography, and the correctness of its structure is also confirmed by nuclear magnetic spectrum.
Embodiment 3
[0051] Embodiment 3: the synthetic method three of triethyl phosphate
[0052]
[0053] Disperse 6.2mg of black phosphorus crystals in 20mL of ethanol, add 5mL of concentrated hydrochloric acid dropwise to the system, stir and react at 60°C for 6h, stop the reaction after the solution is clear, then remove the solvent by rotary evaporation, and obtain yellow Oily product triethyl phosphate, its yield is 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com