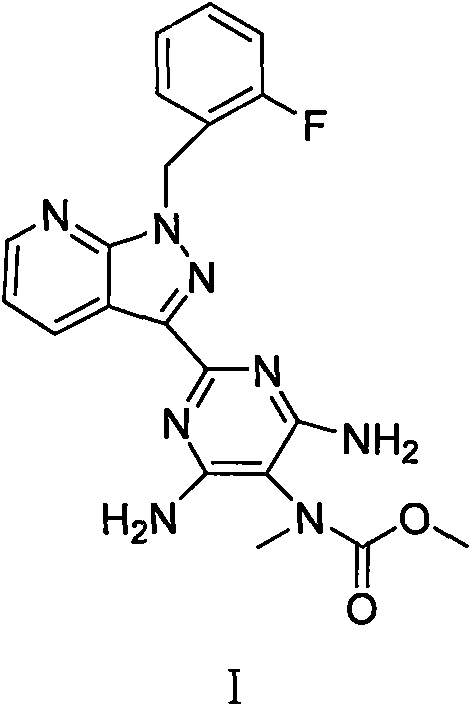
Synthesis method of riociguat
A synthesis method and compound technology, applied in organic chemistry and other directions, can solve problems such as being unsuitable for industrial production, reducing product generation, difficult post-processing, etc., and achieving favorable storage and subsequent reactions, convenient post-processing operations, and good chemical stability. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
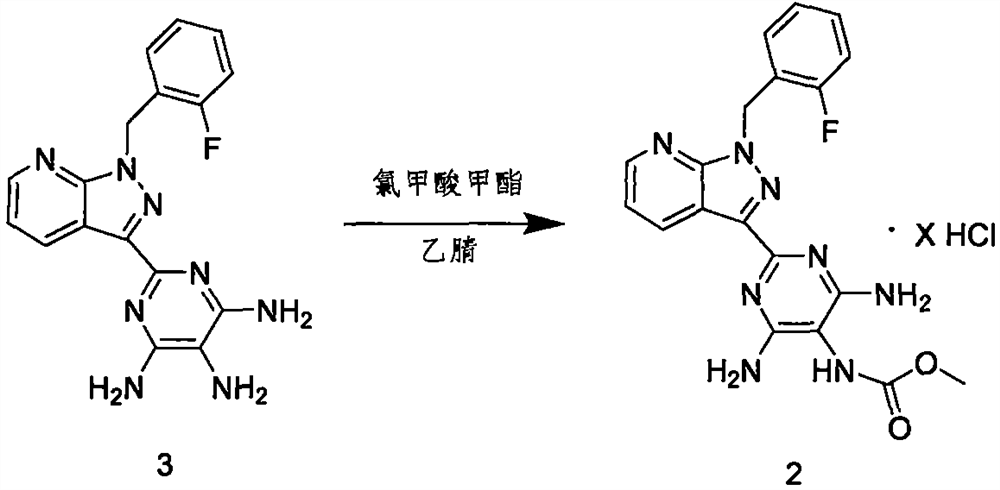
[0035] step 1
[0036] Under nitrogen protection, 20.0g (57.0mmol) of compound 3 was added to 300mL of acetonitrile, stirred, and 8.1g (86.0mmol) of methyl chloroformate was added at room temperature, the temperature was raised to 60°C, and the reaction was stirred for 2 hours, and the reaction was monitored by HLPC end. After the reaction was completed, the reaction liquid was cooled to room temperature, and the unreacted methyl chloroformate was destroyed, filtered by suction, stirred with 200 mL of acetonitrile, dried by suction, and dried by blowing air to obtain 25.7 g of a yellow solid.
[0037] step 2
[0038]Dissolve 20.0g (43.3mmol) of the intermediate product synthesized in the previous step in 100mL of N,N-dimethylformamide, stir, add 64.0g (86.6mmol) of lithium carbonate in batches, control the temperature at 30±5°C, and stir 8.0 g (56.3 mmol) of methyl iodide were added at the same time. The temperature was controlled at 30±5°C, and after stirring for 2 hours, ...
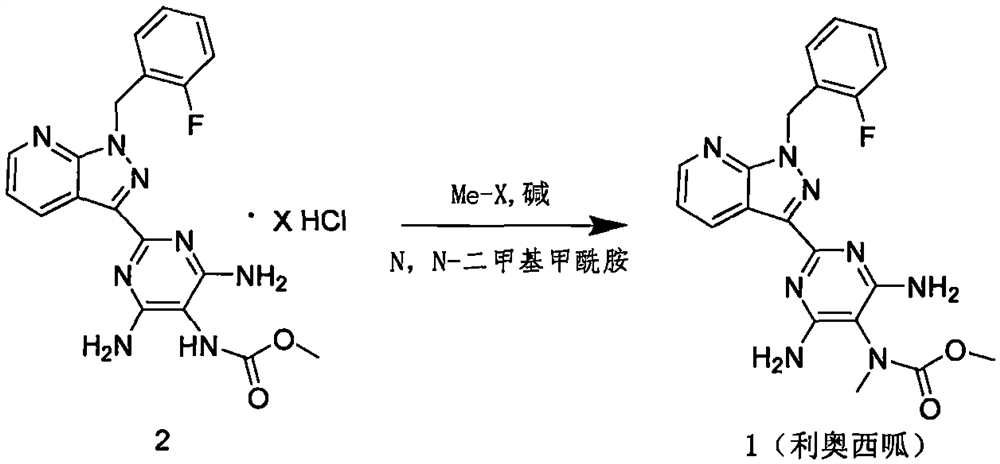
Embodiment 2
[0042] step 1
[0043] Under the protection of nitrogen, 1200g (3.42mol) of compound 3 was added to 1800mL of acetonitrile, stirred, and 486.0g (6.88mol) of methyl chloroformate was added at room temperature, the temperature was raised to 60°C, and after stirring for 2 hours, the reaction endpoint was monitored by HLPC. After the reaction, cool down to room temperature, add methyl chloroformate destroying reagent, stir, filter with suction, wash with 1200mL of acetonitrile, dry with suction, and blow dry to obtain 1536g of yellow solid.
[0044] step 2
[0045] Dissolve 1500.0g (3.25mol) of the intermediate product synthesized in the previous step in 750mL of N,N-dimethylformamide, add 4808g (6.5mol) of lithium carbonate in batches, control the temperature at 30±5°C, and add 600.0g (4.22mol) methyl iodide, temperature controlled at 30±5°C, after stirring for 2 hours, HPLC monitored the reaction end point, after the reaction was completed, add 8.25L of water, stir for 30min, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com