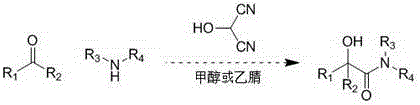
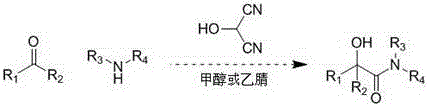
Method for synthesizing α-hydroxyamides by 2-hydroxymalonocyanide
A technology of hydroxymalonocyanide and hydroxyamide, which is applied in the formation/introduction of amide groups, the preparation of carboxylic acid amides, chemical instruments and methods, etc., can solve the problems of high cost, long reaction time, complicated operation and post-treatment, etc. To achieve the effect of short reaction time, clean reaction and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016] Benzaldehyde (106 mg, 1 mmol) and methylamino alcohol solution (470 mg, 33% mass fraction, 5 mmol) were dissolved in 2 mL of methanol, and acetic acid solution of 2-hydroxypropanocyanide (5 mL, 1 mol / L) was added, 50 Stir for 10 minutes at °C. The reaction solution was concentrated and separated by column chromatography to obtain α-hydroxyphenylacetamide (139 mg, 84%). 1 H NMR (400 MHz, CD 3 OD)δ 7.43-7.26 (m, 5H), 5.17 (s, 1H), 3.68 (s, 3H).
Embodiment 2
[0018]
[0019] 2-Formaldehyde furan (96 mg, 1 mmol) and methylamino alcohol solution (940 mg, 33% mass fraction, 10 mmol) were dissolved in 2 mL of methanol, and 2-hydroxypropanedicyanide in trifluoroacetic acid solution (0.1 mL, 10 mol / L), stirred at 30°C for 100 minutes. The reaction solution was concentrated and separated by column chromatography to obtain a-hydroxy-2-furacetamide (128 mg, 83%). 1 HNMR (400 MHz, CD 3 OD) δ 8.20-8.15 (m, 1H), 7.55-7.54 (m, 1H), 6.53-6.44 (m, 2H), 5.80 (s, 1H), 3.68 (s, 3H).
Embodiment 3
[0021]
[0022] Trans-cinnamaldehyde (132 mg, 1 mmol) and methylamino alcohol solution (94 mg, 33% mass fraction, 1 mmol) were dissolved in 2 mL of methanol, and acetic acid solution of 2-hydroxypropanedicyanide (0.4 mL, 7.75 mol / L), stirred at 0°C for 120 minutes. The reaction solution was concentrated and separated by column chromatography to obtain trans-methyl 2-hydroxy-3-ene-phenylbutyramide (167 mg, 87%). 1 H NMR (400 MHz, CD 3 OD) δ 7.48 (d, J= 1.2 Hz, 2H), 7.40-7.34 (m, 3H),7.02 (d, J= 15.6 Hz, 1H), 6.18-6.11 (m, 1H), 4.72 (d, J = 9.2 Hz, 1H), 3.87(s, 3H), 2.71 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com