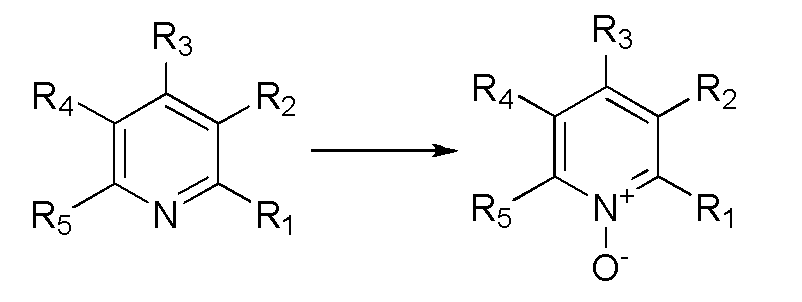
Synthetic method for preparing pyridine N-oxide
A technology of pyridine nitrogen oxide and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of difficult post-processing, unsuitable industrial production, and high raw material prices, and achieve the effects of improving production safety factor, easy operation, and small environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
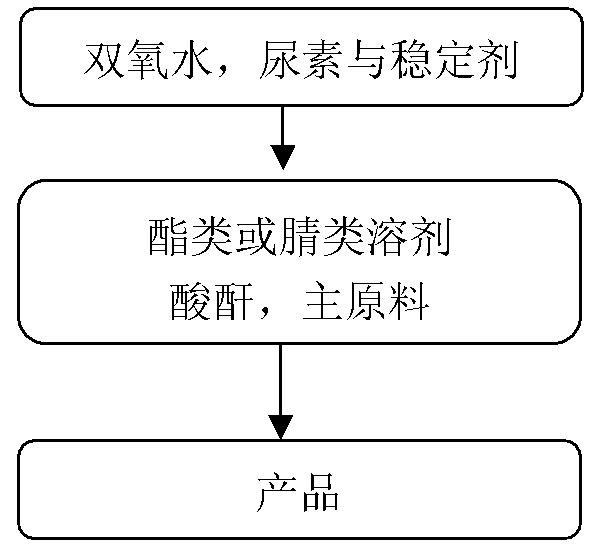
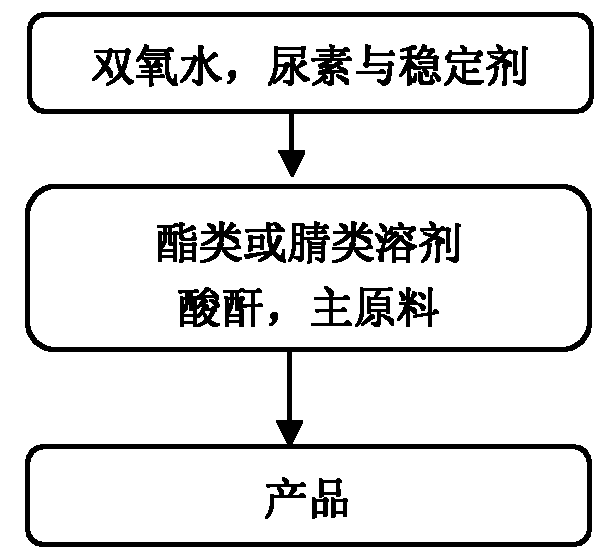
[0029] (1) Preparation of urea-hydrogen peroxide complex: add 708.2kg 30% hydrogen peroxide (3eq.), 12.5kg salicylic acid (0.1g / g) and 125kg urea (1.0eq.) to a 2000L enamel kettle, and heat Insulation reaction. After the reaction is complete, cool down to -10-0°C, heat and stir for 3 hours and then centrifuge. The centrifuged solid is rinsed with 375L of petroleum ether (3ml / g), and the filter cake is a urea-hydrogen peroxide complex with a yield of 80% and a purity of 98%.
[0030] (2) Preparation of product 2-chloropyridine-N-oxide The reaction process: 360L ethyl acetate (3ml / g), 155kg phthalic anhydride (1.0eq.), and 120kg dichloropyridine (1.0eq.) were added to a 2000L enamel kettle. After the feeding is complete, raise the temperature of the system to 90-110°C, add 200kg of urea-hydrogen peroxide compound (2.0eq) to the system, and keep it warm; The test paper no longer turns blue, and then adjust the pH of the system to 9-10 with 20% sodium hydroxide, centrifuge, ext...
Embodiment 2
[0035] (1) Preparation of urea-hydrogen peroxide compound: add 47.2kg 30% hydrogen peroxide (0.5eq.), 4.0kg ethylenediaminetetraacetic acid (0.08g / g) and 50kg urea (1.0eq.) in 300L enamel kettle, ~40°C heat preservation reaction. After the reaction is complete, lower the temperature to 5-15°C, keep stirring for 1 hour and then centrifuge. The centrifuged solid is rinsed with 25L isopropyl acetate (0.5ml / g), and the filter cake is urea-hydrogen peroxide complex, with a yield of 78% and a purity of 98%. ;
[0036] (2) Preparation of product 2-methoxypyridine-N-oxide The reaction process of: add 18L isopropyl acetate (15ml / g) in 72L reaction bottle, 3.3kg acetic anhydride (4eq.), 1.2kg 2-methoxypyridine (1.0eq), after feeding completes, system is warmed up to 30~40℃, add 2.8kg urea hydrogen peroxide compound (4.5eq) to the system, and keep warm for reaction; after the reaction is complete, lower the temperature to 0~10℃, add 0.12kg saturated sodium sulfite solution (until the ...
Embodiment 3
[0041] (1) Preparation of urea-hydrogen peroxide compound: add 75.6kg 30% hydrogen peroxide (0.8eq.), 0.05kg potassium dihydrogen phosphate (0.001g / g) and 50kg urea (1.0eq.) to a 200L enamel kettle, 40 ℃ insulation reaction. After the reaction is complete, lower the temperature to 0-5°C, heat and stir for 0.5 h, then centrifuge, and rinse the centrifuged solid with 100L ethyl acetate (2ml / g). The filter cake is the urea-hydrogen peroxide complex, with a yield of 79% and a purity of 97%;
[0042] (2) Preparation of product 2-chloro-3-picoline nitrogen oxide The reaction process: add 252L ethyl acetate (9ml / g) in the 500L enamel kettle, 45.7kg acetic anhydride (2.5eq.), 28kg 2-chloro-3-picoline (1.0eq.), after the addition is complete, the The temperature of the system is raised to 80-110°C, and 16.8kg of urea-hydrogen peroxide complex (1.0eq) is added to the system, and the reaction is carried out with heat preservation; after the reaction is complete, the temperature is lowe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com