Preparation method of hydrazine hydrate
A technology of hydrazine hydrate and hydrazine salt, which is applied in the directions of hydrazine, nitrogen and non-metallic compounds, can solve the problems of reducing the yield of hydrazine hydrate, affecting the quality of hydrazine hydrate, and high equipment requirements, so as to save the refining process and equipment investment, The production process is safe and stable, and the effect of improving purity and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
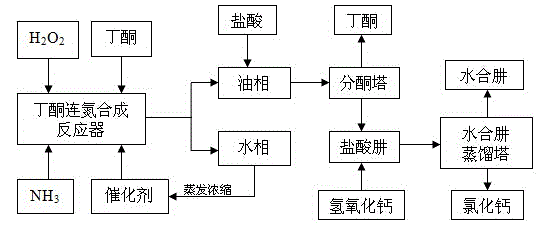
[0040] refer to figure 1 process for production. 108g of butanone, 34g of formamide and 170g of mass fraction of 20% ammonia are packed into a four-necked flask with a reflux condenser, a thermometer, a dropping funnel and stirring, and a mass fraction of 30% peroxide is added in the dropping funnel. Hydrogen 56.7 g (0.5 mol). Heat in a water bath to raise the temperature of the solution to 45°C, start adding hydrogen peroxide dropwise, control the reaction temperature to 45°C, and complete the dropwise in 3 to 4 hours. After the dropwise addition, the temperature was raised to 50° C. and kept at 50° C. to continue the reaction for 2 hours. After completion of the reaction, stop heating and stirring, and transfer the reaction liquid into a separatory funnel after cooling, and separate the water phase and the oil phase, the content of butanazine in the oil phase is 69.2%. The aqueous phase is distilled and concentrated to recover ammonia, methyl ethyl ketone and catalyst. T...
Embodiment 2
[0042] 1100g butanone, 350g formamide and 1360g mass fraction of 28% ammonia water are charged into a jacketed reactor with stirring and reflux condenser, and there is a refrigerant for controlling the reaction temperature in the jacket. The temperature of the solution was raised to 40°C, and 566.7g of hydrogen peroxide (30%) was added dropwise, the refrigerant was turned on, the reaction temperature was controlled to be 40°C, and the dripping was completed in 3 to 4 hours. After the dropwise addition, the temperature was raised to 45°C, and the reaction was continued at 45°C for 2.5 hours. After completion of the reaction, stop heating and stirring, transfer the reaction solution into a separatory funnel, separate the water phase and the oil phase, and the content of butanazine in the oil phase is 67.7%. The aqueous phase is distilled and concentrated to recover ammonia, methyl ethyl ketone and catalyst. The oil phase is transferred to a jacketed reactor with stirring and dist...
Embodiment 3
[0044] 150g butanone, 52g ammonium acetate, 63g acetamide and 156g mass percent concentration are 25% ammoniacal liquor to pack in the four-neck flask that band reflux condenser, thermometer, dropping funnel and stir, add mass fraction in dropping funnel. 37.8g (1mol) of 90% hydrogen peroxide. Heat in a water bath to raise the temperature of the solution to 30°C, start adding hydrogen peroxide dropwise, control the reaction temperature to 30°C, and complete the dropwise in 3 to 4 hours. After the dropwise addition, the temperature was raised to 45° C. and kept at 45° C. to continue the reaction for 3 hours. After the reaction, stop heating and stirring, transfer the reaction solution into a separatory funnel, separate the water phase and the oil phase, and the content of butanazine in the oil phase is 95.5%. The aqueous phase is distilled and concentrated to recover ammonia, methyl ethyl ketone and catalyst. Transfer the oil phase into a four-neck flask with distillation con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com