Method of joint production of urea hydrogen peroxide and sodium percarbonate
A technology of urea peroxide and sodium percarbonate, applied in the direction of chemical instruments and methods, peroxide/peroxyhydrate/peroxyacid/superoxide/ozonide, organic compound preparation, etc. To avoid problems such as other methods of urea peroxide, hydrogen peroxide waste, and low production costs, to avoid adverse effects, mild reaction conditions, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
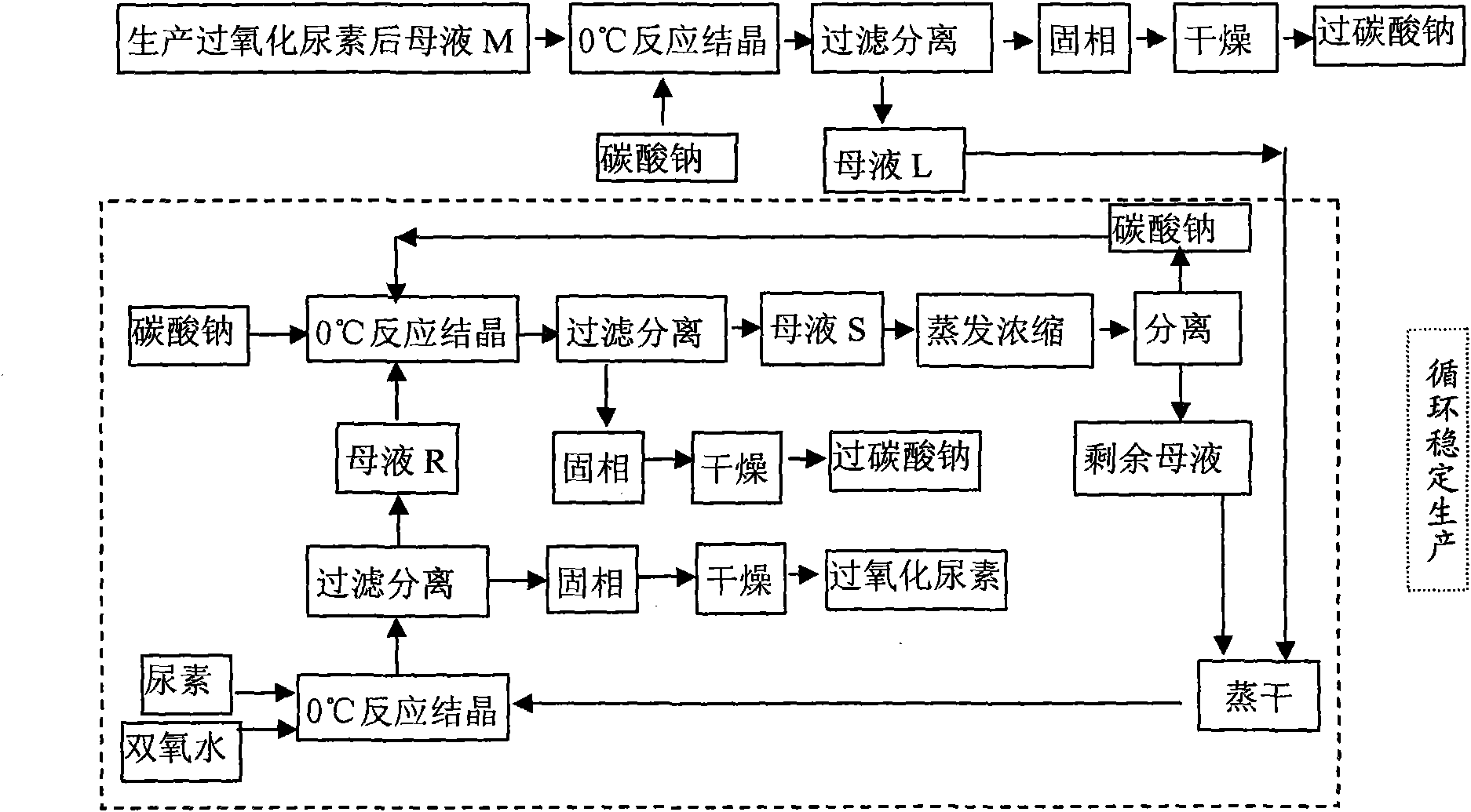
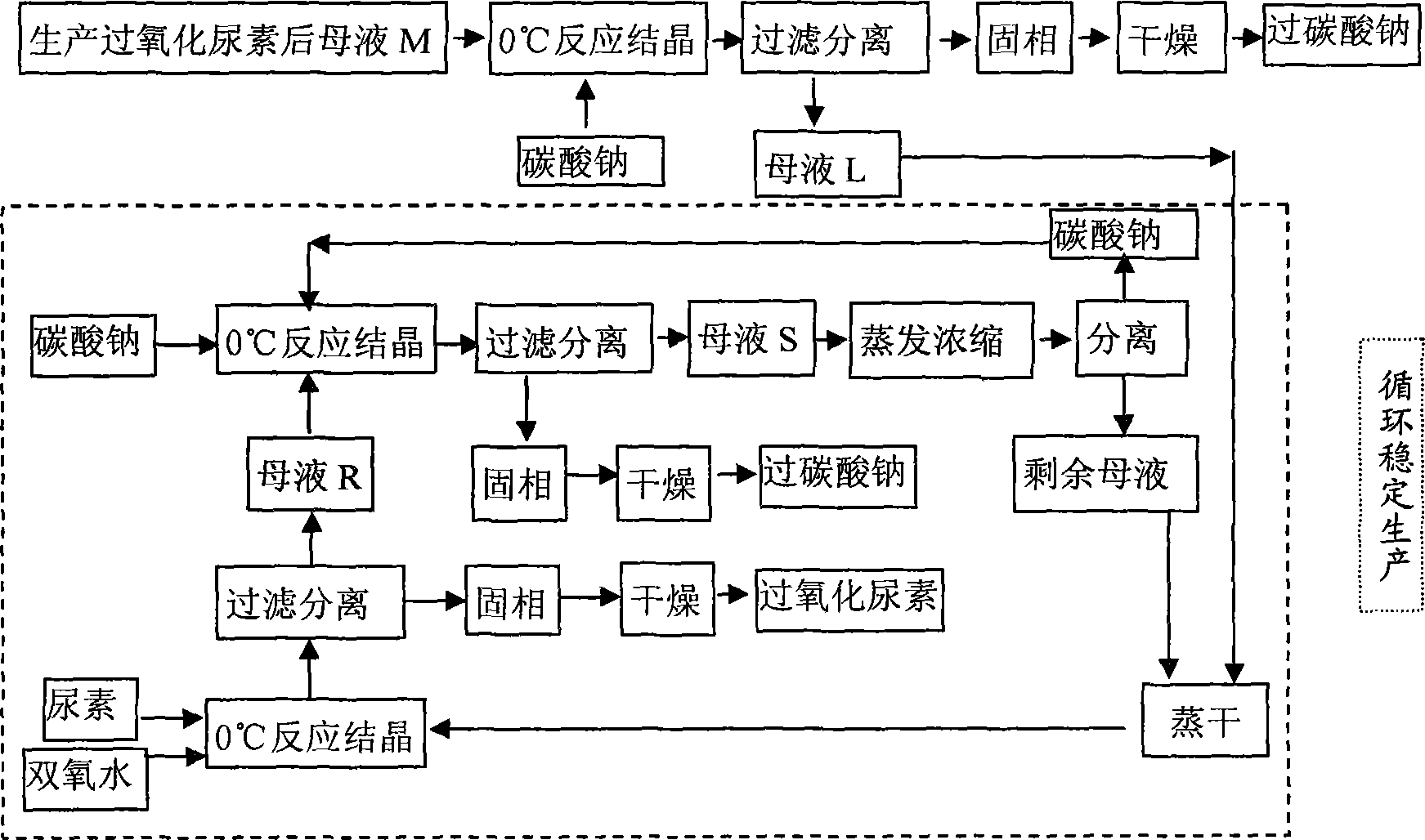
[0029] Example 1 (process route sees figure 1 )
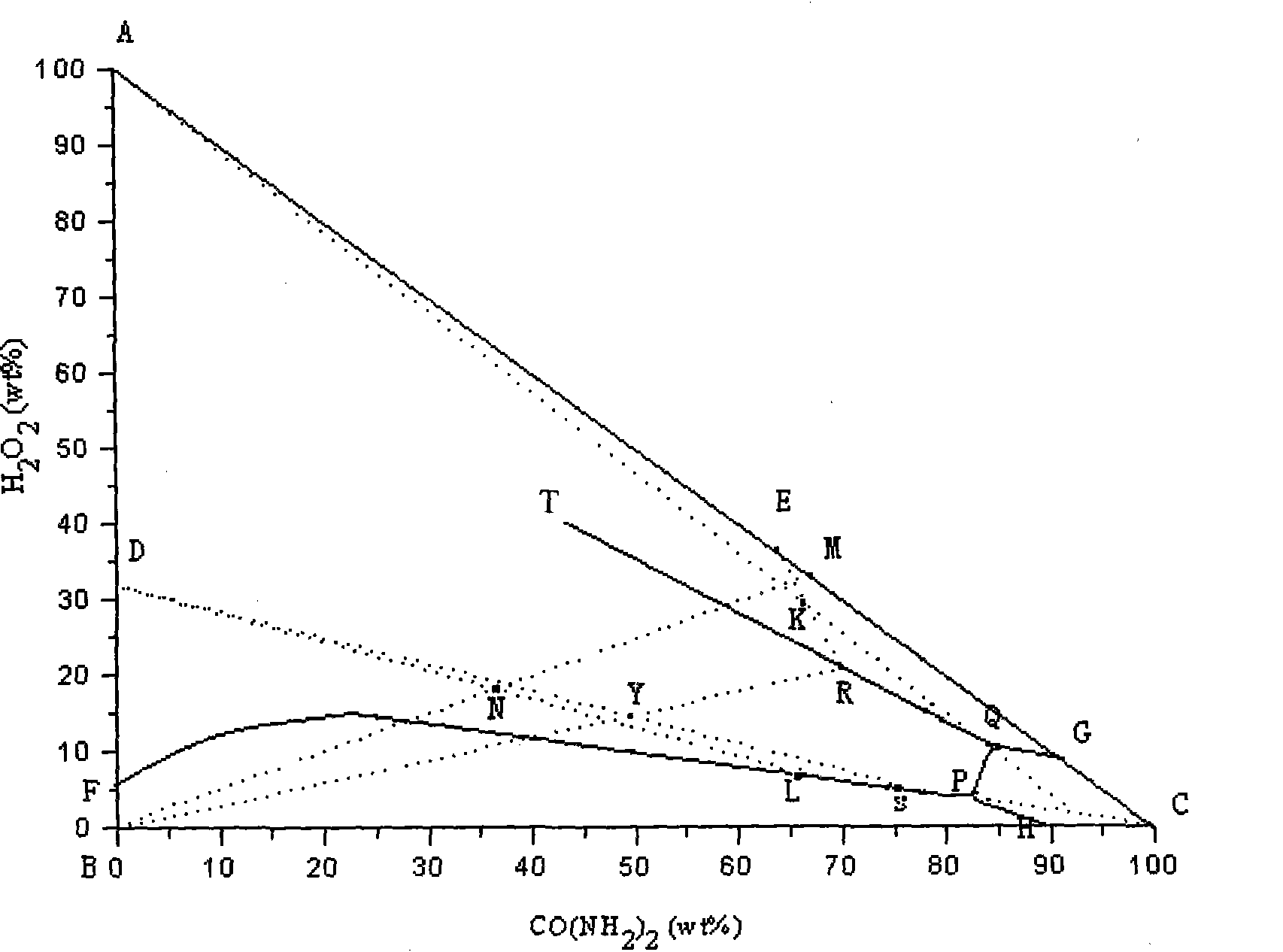
[0030] (1) Take 1700g of hydrogen peroxide with a mass concentration of 30% and add it to a 3L three-necked flask, then add 939g of CO(NH 2 ) 2 (Analytical pure) crystals, mechanically stirred and reacted in a constant temperature bath at 0°C, after 120 minutes, urea peroxide crystals will crystallize and precipitate, centrifuge, and dry in a solid phase oven at 60°C to obtain 1018g of white flaky crystal product urea peroxide . Gained mother liquor is that M is 1619g, records H in M mother liquor 2 o 2 The mass percentage content is 8.73%, CO(NH 2 ) 2 The mass percentage content of the hydrogen peroxide is 17.78% (mass ratio is hydrogen peroxide: urea: water=1: 2.04: 8.42).
[0031] (2) Add the above 1619g M mother liquor into a 2L three-necked flask, then add 4.25g water glass, 2.55g magnesium chloride, 0.50g EDTA (as a stabilizer), and add anhydrous Na 2 CO 3 355g (Since water glass, magnesium chloride, EDTA are a...
example 2
[0036] Step (1) (2) is the same as embodiment 1
[0037] (3) Evaporate the mother liquor L obtained in (2) to dryness (all hydrogen peroxide is lost therein), join it in the 5L three-necked flask, then add 1506g CO(NH 2 ) 2 , 2538g mass concentration is 30% H 2 o 2 Solution, after the solid phase is fully dissolved, mechanically stir the reaction in a constant temperature tank at 0°C, and after 60 minutes, urea peroxide crystals will crystallize and precipitate. After being centrifuged by a centrifuge, the solid phase is then placed in an oven at 60°C for drying to obtain 1378 g of white flaky crystal product urea peroxide. Gained filtrate R is 3074g, records H in mother liquor R 2 o 2 The mass percentage content is 8.57%, CO(NH 2 ) 2 The mass percentage content is 29.75%, Na 2 CO 3 The mass percentage content is 3.88%.
[0038] (4) Add the mother liquor R obtained in (3) into a 5L three-neck flask, add 481gNa 2 CO 3 , After the sodium carbonate is fully dissolved,...
example 3
[0041] Step (1) (2) is with embodiment 1;
[0042] (3) Evaporate the mother liquor L obtained in (2) to dryness (all the hydrogen peroxide in it is lost), put it into a 5L three-necked flask, and then add 1500.73gCO (NH 2 ) 2 , 2566.39g mass concentration of 30% H 2 o 2 Solution, after the solid phase is fully dissolved, mechanically stir the reaction in a constant temperature tank at 0°C, and after 60 minutes, urea peroxide crystals will crystallize and precipitate. After centrifugation in a centrifuge, the solid phase is put into an oven at 60° C. for drying to obtain 1395 g of white flaky crystal product urea peroxide. The resulting mother liquor R is 3079.66g, and the H in the R mother liquor is measured 2 o 2 The mass percentage content is 8.63%, CO (NH 2 ) 2 The mass percentage content is 29.17%, Na 2 CO 3 The mass percentage content is 3.88%.
[0043] (4) Add the R mother liquor obtained in (3) into a 5L three-necked flask, add 484gNa 2 CO 3 , After the sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com