Polymers, phosphorescent host materials and electroluminescent devices
A technology of electroluminescent devices and phosphorescent hosts, applied in phosphorescent host materials and electroluminescent devices, polymers with functional groups as side groups, and polymers to achieve high device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
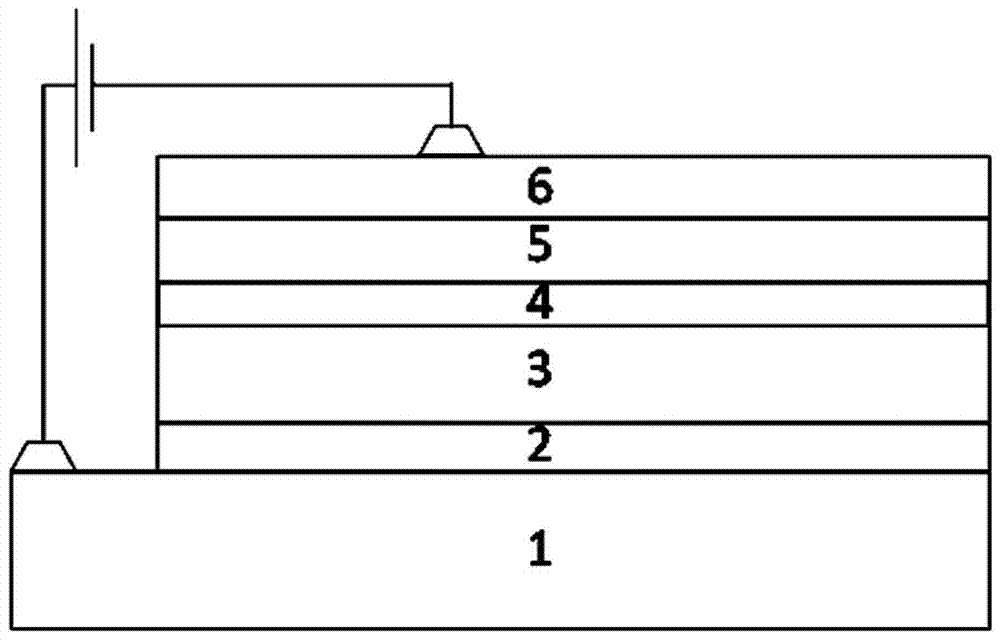
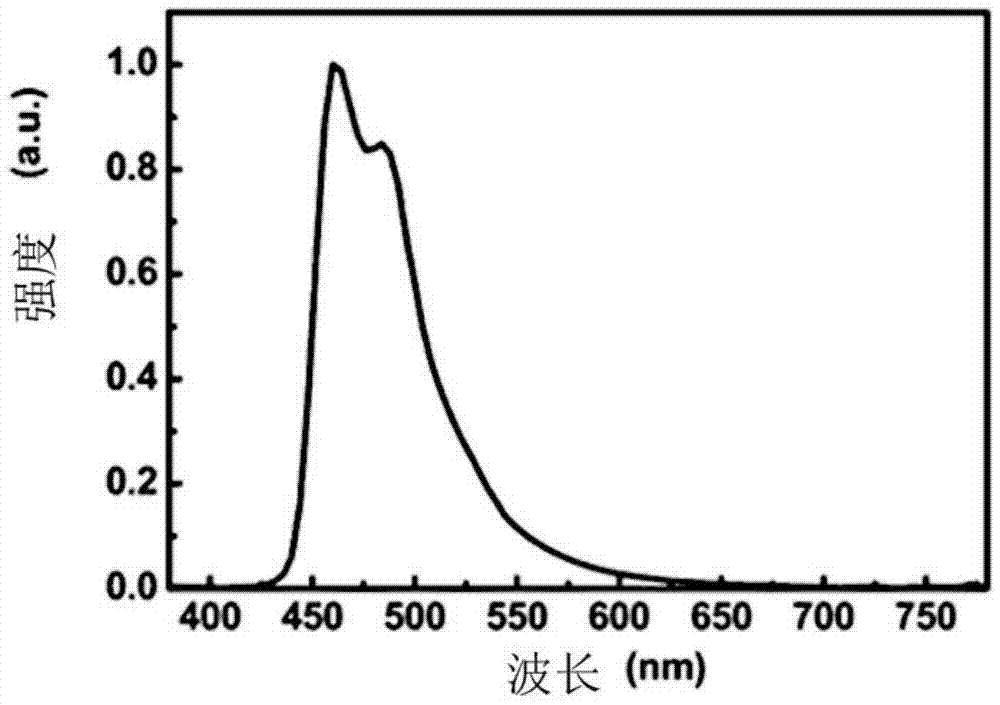
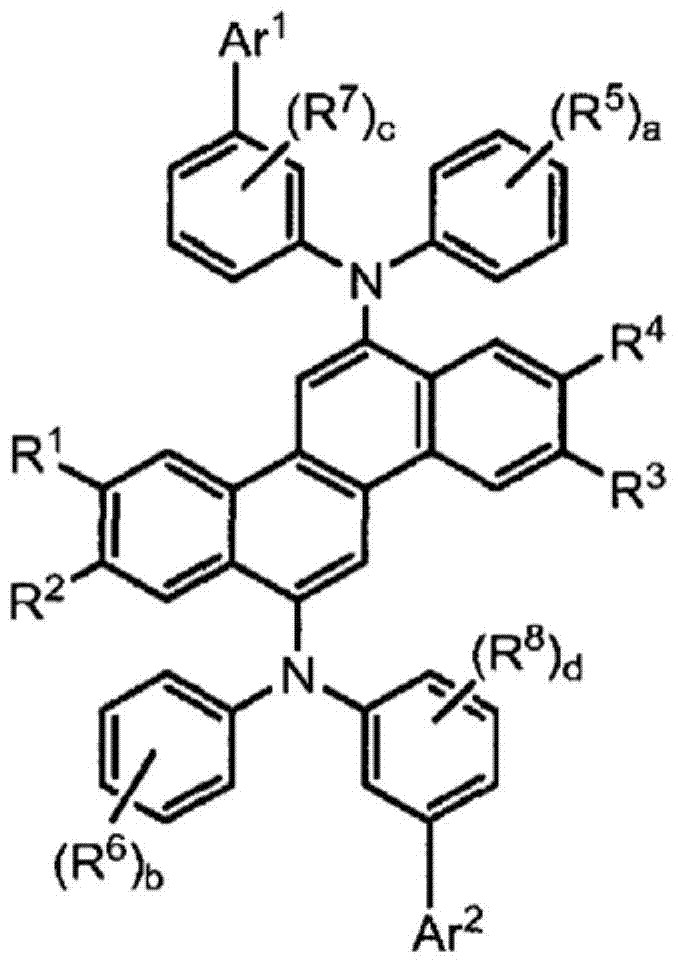
Image
Examples
preparation example 1
[0090]Preparation of 4-(diethoxy(methyl)silyl)-N,N-diphenylamine (abbreviated as monomer 1):
[0091]
[0092] Under argon atmosphere, 4-bromophenyl-N,N-diphenylamine (6 mmol) was dissolved in 200 mL of anhydrous THF. After the temperature in the bottle dropped to -78°C, 3.3 ml of n-butyllithium (2.2M in n-hexane solvent) was added dropwise within 15 minutes. The reaction was continued for half an hour while maintaining the system temperature, and then 1.5 g (8.9 mmol) of methyldiethoxychlorosilane was rapidly added dropwise. After the dropwise addition, the reaction was continued to gradually rise to room temperature, and then quenched with ammonium chloride solution. The reactant was extracted with ether, washed three times with water and dried over anhydrous magnesium sulfate. The filtrate was concentrated and subjected to silica gel column chromatography (petroleum ether:dichloromethane=6:1) to obtain a colorless oily product with a yield of 42%.
[0093] 1 H NMR (4...
preparation example 2
[0098] Preparation of (4-(diethoxy(methyl)silyl)phenyl)diphenylphosphine oxide (abbreviated as monomer 2):
[0099]
[0100] (4-Bromophenyl)-diphenylphosphine oxide (6 mmol) was dissolved in 200 mL of anhydrous THF under argon atmosphere. After the temperature in the bottle dropped to -78°C, 3.3 ml of n-butyllithium (2.2M in n-hexane solvent) was added dropwise within 15 minutes. The reaction was continued for half an hour while maintaining the system temperature, and then 1.5 g (8.9 mmol) of methyldiethoxychlorosilane was rapidly added dropwise. After the dropwise addition, the reaction was continued to gradually rise to room temperature, and then quenched with ammonium chloride solution. The reactant was extracted with ether, washed three times with water and dried over anhydrous magnesium sulfate. The filtrate was concentrated and subjected to silica gel column chromatography (petroleum ether:dichloromethane=6:1) to obtain a colorless oily product with a yield of 63%. ...
preparation example 3-4
[0106] The methyldiethoxychlorosilane of Preparation Example 1 or 2 is replaced by phenyldiethoxychlorosilane to obtain 4-(diethoxy(phenyl)silyl)-N,N-diphenylamine ( Abbreviated as monomer 3) (4-(diethoxy(phenyl)silyl)phenyl)diphenylphosphine oxide (abbreviated as monomer 4).
[0107] Monomer 3:
[0108] 1 H NMR (400MHz, CDCl 3 ,δ):0.37(s,3H,-CH 3 ), 1.27(t, J=7Hz, 6H; -OCH 2 CH 3 ), 3.86 (q, J=7Hz, 4H; -OCH 2 ), 7.06(d, J=8Hz, 4H; ArH), 7.13(d, J=7Hz, 4H; ArH), 7.32(m, 9H; ArH), 7.48(d, J=2Hz, 4H; ArH ).
[0109] 13 C NMR (400MHz, CDCl 3 ,δ): -1.9, 20.5, 60.5, 124.1, 125.3, 126.6, 129.4, 131.4, 136.5, 137.1, 148.5, 151.1.
[0110] 29 Si-NMR (400MHz, CDCl 3 ,δ): -17.2.
[0111] Elemental analysis theoretical value C28H29NO2Si: C, 76.50; H, 6.65; N, 3.19. Actual value: C, 76.43; H, 6.61; N, 3.12.
[0112] Monomer 4:
[0113] 1H NMR (400MHz, CDCl3, δ): 0.38 (s, 3H, -CH3), 1.33 (t, J = 7Hz, 6H; -OCH2CH3), 3.86 (q, J = 7Hz, 4H; -OCH2), 7.36 ( brs, 17H; Ar H), 7.68 ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap