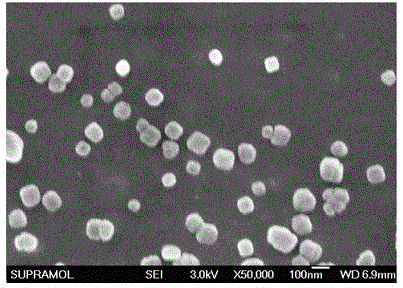
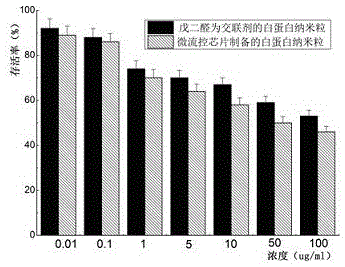
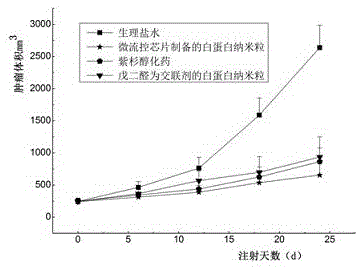
Taxane drug albumin nanoparticle freeze-drying preparation for injection and preparation method
A technology of albumin nanoparticles and taxanes, applied in the field of pharmaceutical preparations, to achieve good dispersion, good application prospects, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 . Preparation of paclitaxel albumin nano-lyophilized preparation.
[0033] (1) Take 20ml of pre-prepared 0.5% potassium chloride solution, mix it with 8ml of human serum albumin, and place them together in a 50°C water bath for incubation as the water phase.
[0034] (2) Accurately weigh 200mg of paclitaxel and dissolve in 10ml of ethanol at a concentration of 20mg / ml as the oil phase.
[0035] (3) Use a peristaltic pump to incubate the water phase in step (1) and keep it at 50°C through a heat exchanger. At the same time, the oil phase in step (2) is also under the action of the peristaltic pump, and the water phase: oil phase pump speed = 2.5 The ratio of :1 enters the three-way structure of the microfluidic device at the same time, and the mixed liquid is rapidly cooled to 0-10°C at the outlet of the three-way.
[0036] (4) Concentrate the solution obtained in step (3) by using a hollow fiber module, add 1000mg of mannitol, and filter through a 0.22um ...
Embodiment 2
[0037] Example 2 . Preparation of paclitaxel albumin nano-lyophilized preparation.
[0038] (1) Take 20ml of pre-prepared 5% potassium chloride solution, mix it with 8ml of human serum albumin, and place them together in a 50°C water bath for incubation as the water phase.
[0039] (2) Accurately weigh 300mg of paclitaxel and dissolve it in 10ml of ethanol at a concentration of 30mg / ml as the oil phase.
[0040] (3) Use a peristaltic pump to incubate the water phase in step (1) and keep it at 70°C through a heat exchanger. At the same time, the oil phase in step (2) is also under the action of the peristaltic pump, and the water phase: oil phase pump speed = 2.5 The ratio of :1 enters the three-way structure of the microfluidic device at the same time, and the mixed liquid is rapidly cooled to 0-10°C at the outlet of the three-way.
[0041] (4) Concentrate the solution obtained in step (3) by using a hollow fiber module, add 1000mg of mannitol, and filter through a 0.22um...
Embodiment 3
[0042] Example 3 . Preparation of docetaxel albumin nano-lyophilized preparation.
[0043] (1) Take 20ml of pre-prepared 15% potassium chloride solution, mix it with 12ml of human serum albumin, and place them together in a 50°C water bath for incubation as the water phase.
[0044] (2) Accurately weigh 300mg of docetaxel and dissolve it in 10ml of ethanol at a concentration of 30mg / ml as the oil phase.
[0045] (3) Use a peristaltic pump to incubate the water phase in step (1) and keep it at 70°C through a heat exchanger. At the same time, the oil phase in step (2) is also under the action of the peristaltic pump, and the water phase: oil phase pump speed = 3 The ratio of :1 enters the three-way structure of the microfluidic device at the same time, and the mixed liquid is rapidly cooled to 0-10°C at the outlet of the three-way.
[0046] (4) Concentrate the solution obtained in step (3) by using a hollow fiber module, add 1000mg of mannitol, and filter through a 0.22um mic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com