Preparation method for high-base-number crystalline calcium sulfonate
A high-alkaline value calcium sulfonate, crystalline technology, applied in the preparation of organic compounds, chemical instruments and methods, petroleum industry, etc., to achieve the effects of strong applicability, high extreme pressure, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
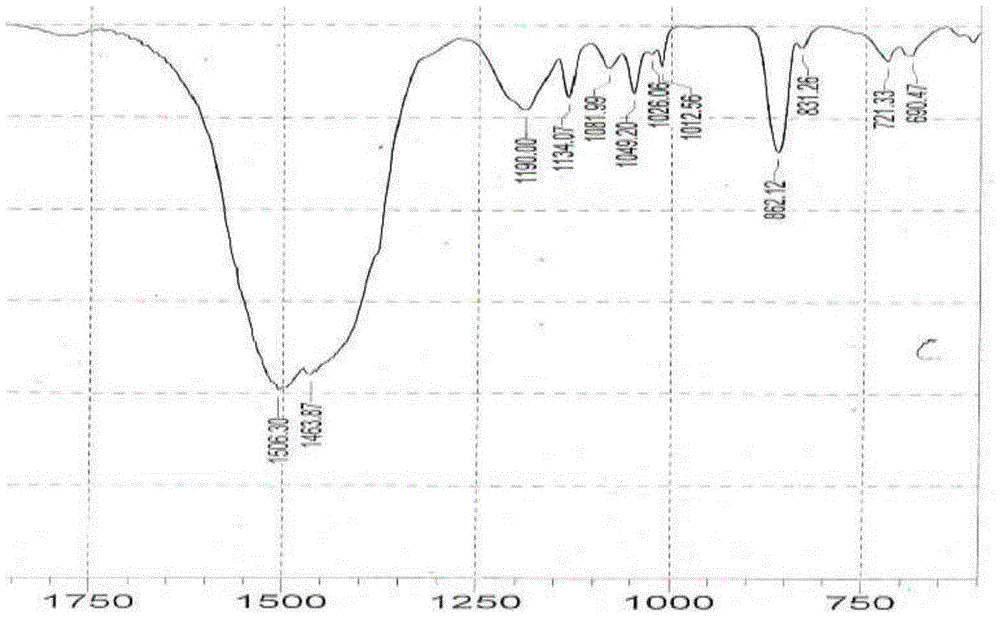
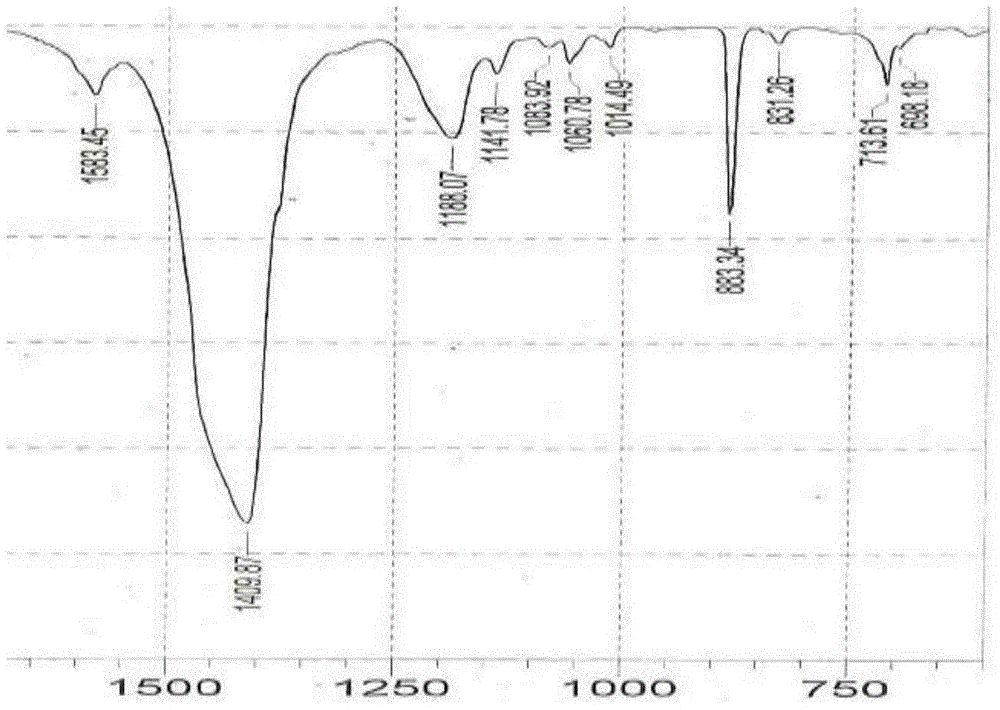
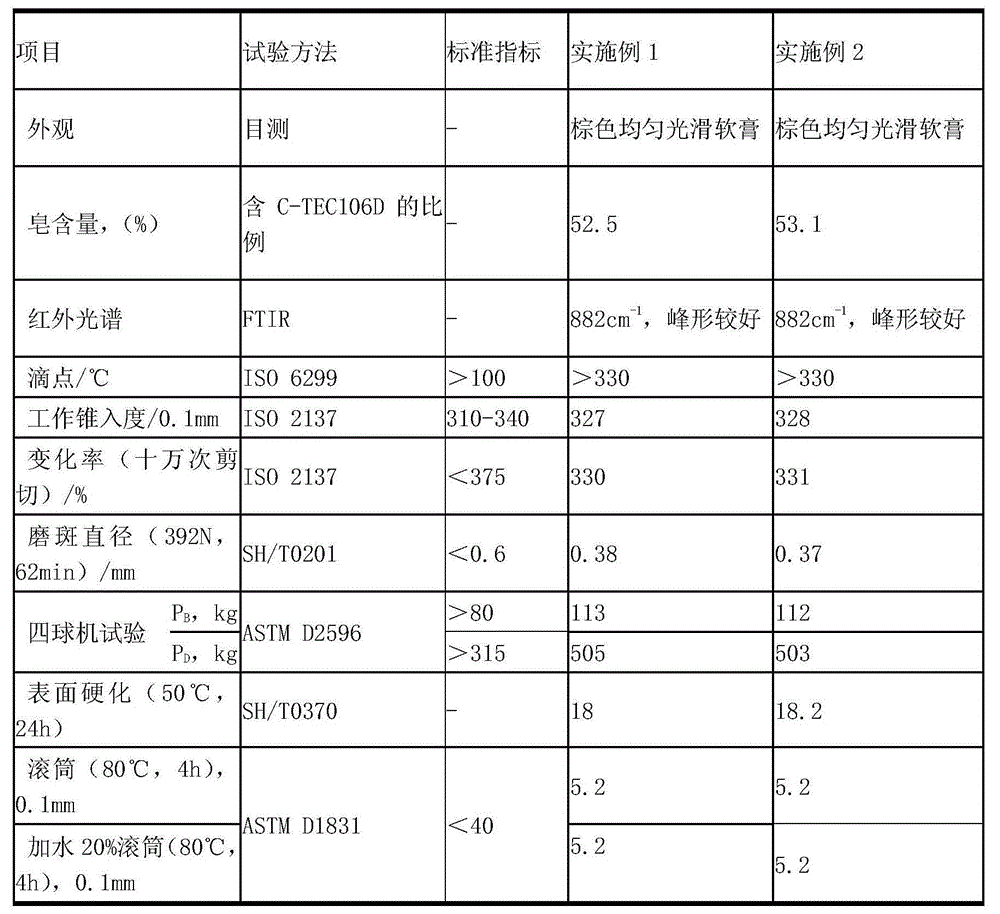
[0025] In a 1-liter reactor, add 200g of amorphous calcium sulfonate with high base value, 20g of methanol, 15g of water and 200g of neutral oil, heat up to 35°C to 40°C, and add calcium sulfonate containing crystalline calcite (calcite content is 20wt%) 6g, mix evenly and seal the reactor, feed carbon dioxide at a rate of 0.12L / min to 0.14L / min, raise the temperature and keep it at 80°C to 85°C until the amorphous All calcium carbonate is converted into calcite crystals; infrared monitoring is carried out during the production process, and the amorphous calcium carbonate reaches 860cm after 2 hours of reaction -1 The peak (amorphous) disappears, all converted to 880cm -1 peaks (calcite), such as figure 1 and figure 2 Stop reaction, steam methanol and water, obtain viscous high-alkali value crystalline (calcite) calcium sulfonate; use this high-alkali value crystalline (calcite) calcium sulfonate for lubricating grease, its various performances are excellent, Its physical ...
Embodiment 2
[0027] In a 1 liter reaction kettle, add 200g of amorphous calcium sulfonate with high base value, 220g of neutral oil, 15g of dodecylbenzenesulfonic acid, 12g of n-butanol and 15g of water, heat up to 45°C to 50°C and stir to mix After uniformity, seal the reactor, feed carbon dioxide at a rate of 0.12L / min to 0.14L / min, raise the temperature and keep it at 75°C to 80°C, and feed carbon dioxide at a rate of 0.12L / min to 0.14L / min until The amorphous calcium carbonate in the high alkali value calcium sulfonate is all converted into calcite crystals; infrared monitoring is carried out during the production process, and the amorphous calcium carbonate reaches 860cm after 3 hours of reaction -1 The peak (amorphous) disappears, all converted to 880cm -1 peaks (calcite), such as figure 1 and figure 2 ;Stop the reaction, evaporate n-butanol and water to obtain viscous high-alkali value crystalline (calcite) calcium sulfonate; use this high-alkaline value crystalline (calcite) cal...
Embodiment 3
[0031] In the reactor of 1 liter, add 200g amorphous calcium sulfonate with high alkali value, 200g neutral oil, 8g dodecylbenzenesulfonic acid, 14g calcium sulfonate containing crystalline calcite (calcite content is 30wt%) , 10g of n-butanol and 15g of water, heat up to 45°C ~ 50°C, stir and mix evenly, seal the reactor, feed carbon dioxide at a rate of 0.12L / min ~ 0.14L / min, raise the temperature and keep it at 75°C ~ 80°C, Until the amorphous calcium carbonate in the high alkali value calcium sulfonate is all converted into calcite crystals. Infrared monitoring is carried out during the production process. After 30 minutes of reaction, the amorphous calcium carbonate is 860cm -1 The peak (amorphous) disappears, all converted to 880cm -1 peaks (calcite), such as figure 1 and figure 2 ;Stop the reaction, evaporate n-butanol and water to obtain viscous high-alkali value crystalline (calcite) calcium sulfonate; use this high-alkaline value crystalline (calcite) calcium sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com