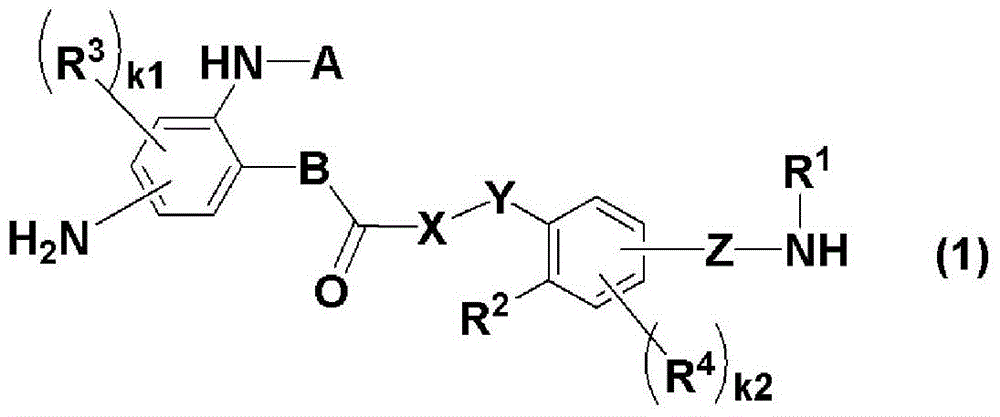
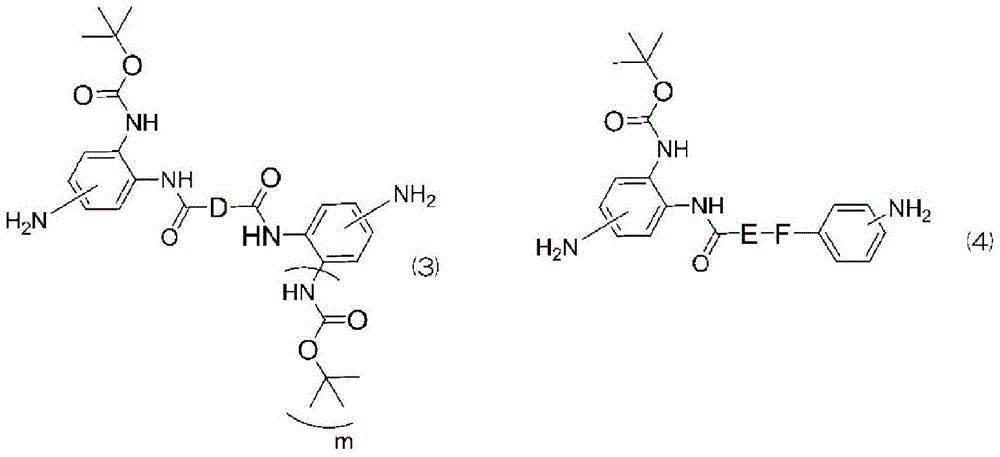
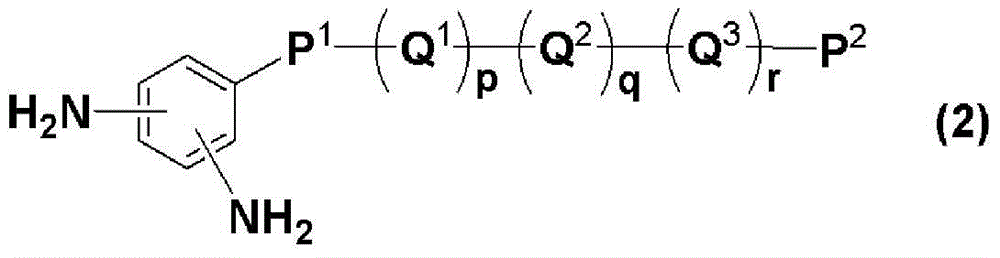New diamine, polymer, liquid crystal aligning agent, liquid crystal aligning film, and liquid crystal display element using the liquid crystal aligning film
A technology of liquid crystal alignment agent and polymer, which is applied in the field of polymers and new diamines. It can solve the problems of unreliable liquid crystal display elements and poor image retention, etc., and achieve good liquid crystal orientation, not easy to accumulate, and good printability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] (Example 1) Synthesis of N1,N4-bis[(2-tert-butoxycarbonylamino)-4-aminophenyl]succinamide [B-6]
[0245]
[0246] Step 14-Synthesis of nitro-2-tert-butoxycarbonylaminoaniline [NB-NA]
[0247] Weigh 200.0 g (1.31 mol) of 4-nitro-1,2-phenylenediamine in a 3L four-necked flask equipped with a stir bar and a nitrogen introduction tube, add 1L of THF and 300 mL of DMF, and heat to about 60 in a nitrogen atmosphere It was dissolved at °C, 285.2 g (1.44 mol) of tert-butyl dicarbonate was slowly added dropwise using a dropping funnel over 2 hours, and it was refluxed for 4 hours.
[0248] After the reaction, the reaction solution was concentrated using a rotary evaporator, the resulting residue was dissolved in THF, and poured into a mixed solvent of ethyl acetate: n-hexane (volume ratio 7:3) for recrystallization, thereby obtaining a yellow Solid 248g (about 75% yield). The resulting solid is 4-nitro-2-tert-butoxycarbonylaminoaniline. Using nuclear magnetic resonance spectroscopy ...
Embodiment 2
[0256] (Example 2) Synthesis of N1,N4-bis[(2-tert-butoxycarbonylamino)-4-aminophenyl]adipamide [B-7]
[0257]
[0258] Step 1 Synthesis of N1, N4-bis[(2-tert-butoxycarbonylamino)-4-nitrophenyl] adipamide
[0259] Weigh 20.0g (78.97mmol) of 2-tert-butoxycarbonylamino-4-nitroaniline and 15.6g (197.43mmol) of pyridine in a 500mL four-neck flask, and dissolve them in a mixed solvent of 300ml dehydrated THF and 100ml DMF. While keeping the bath below 10°C, a THF solution (20% by weight) of 6.5 g (35.54 mmol) of adipoyl chloride was slowly added dropwise using a dropping funnel under a nitrogen atmosphere, and the reaction was stirred at room temperature for 24 hours. As the reaction progressed, a solid precipitated out. After the reaction, 300 ml of pure water was added to the reaction solution and stirred for a period of time, and the reaction solution was poured into 1 L of methanol and stirred for a period of time. The solid was filtered and washed with 500 ml of methanol several t...
Embodiment 3
[0264] (Example 3) Synthesis of N1,N4-bis[(2-tert-butoxycarbonylamino)-4-aminophenyl]terephthalamide [B-8]
[0265]
[0266] Step 1 Synthesis of N1,N4-bis[(2-tert-butoxycarbonylamino)-4-nitrophenyl]terephthalamide
[0267] Weigh 20.0g (78.97mmol) of 2-tert-butoxycarbonylamino-5-nitroaniline and 15.6g (197.43mmol) of pyridine in a 500mL four-necked flask, and dissolve them in a mixed solvent of 300ml dehydrated THF and 100ml DMF. While keeping the bath below 10°C, a THF solution (20% by weight) of 7.2 g (35.54 mmol) of terephthaloyl chloride was slowly added dropwise using a dropping funnel under a nitrogen atmosphere, and the reaction was stirred at room temperature for 24 hours. As the reaction progressed, a solid precipitated out. After the reaction, 300 ml of pure water was added to the reaction solution and stirred for a period of time, and the reaction solution was poured into 1 L of methanol and stirred for a period of time. The solid was filtered and washed with 500 ml of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com