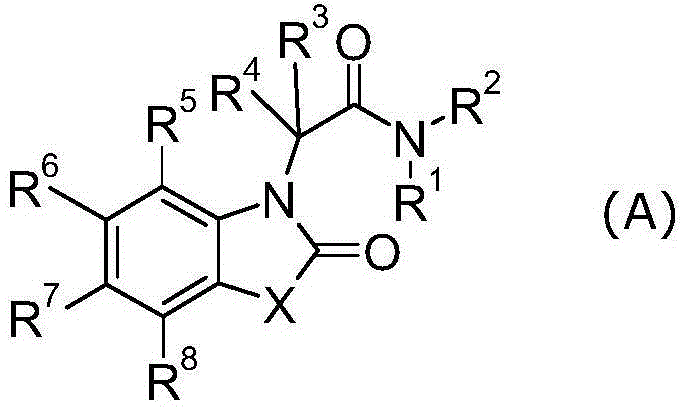
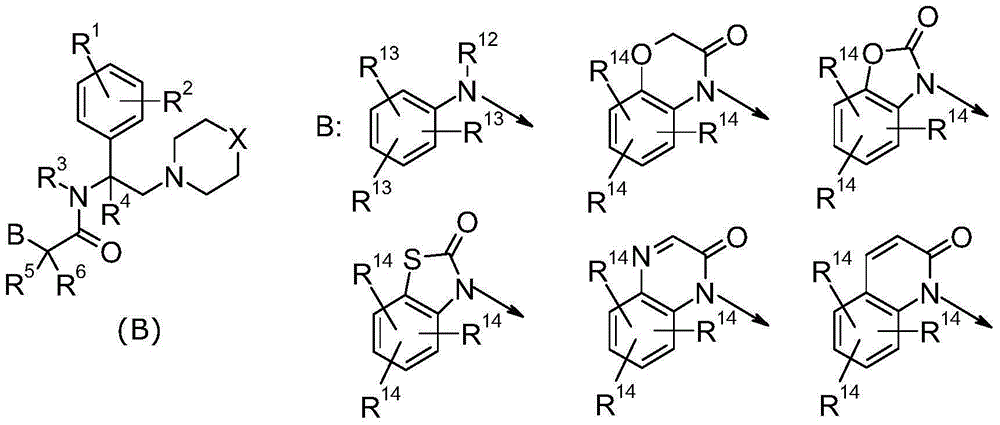
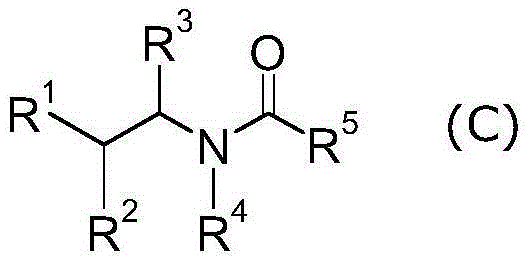
Heterocyclic acetic acid amide compound
A compound and pharmaceutical composition technology, applied in the field of heterocyclic acetamide compounds, can solve problems such as no specific disclosed compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0240] Hereinafter, the method for producing the compound of formula (I) will be described in more detail based on examples. In addition, the present invention is not limited to the compounds described in the following Examples. In addition, the production method of the raw material compound is shown in the production example. In addition, the production method of the compound of formula (I) is not limited to the production method shown in the following specific examples, and the compound of formula (I) can also be produced by a combination of these production methods or by a method obvious to those skilled in the art .
[0241] The following abbreviations are sometimes used in Examples, Production Examples, and Tables described below.
[0242] PEx=production example number, Ex=example number, Syn=example number produced by the same method, PSyn=production example number produced by the same method, Str=structural formula, DAT=physicochemical data, APCI+=APCI- m / z value in ...
manufacture example 1
[0248] Under argon atmosphere, to 5-chloro-1,3-benzo To a mixture of oxazol-2(3H)-one (50.0 g) and acetone (750 mL) was added K at room temperature 2 CO 3 (61.1g) and tert-butyl bromoacetate (52.3mL), heated to reflux for 1.5 hours. The reaction mixture was filtered hot, washing with acetone. The filtrate and washings were combined and concentrated under reduced pressure. The resulting solid was stirred in a mixed solvent of Hex / EtOAc (6 / 1) and recovered by filtration, and further stirred in water and recovered by filtration to obtain (5-chloro-2-oxo-1,3-benzo tert-butyl azol-3(2H)-yl)acetate (81.1 g).
manufacture example 2
[0250] In (5-chloro-2-oxo-1,3-benzo TFA (67.4 mL) was added to a mixture of tert-butyl azol-3(2H)-acetate (50 g) and dichloromethane (250 mL), followed by stirring at room temperature overnight. After the reaction mixture was concentrated under reduced pressure, water was added to the residue. After the solid produced was recovered by filtration, it was washed with water to obtain (5-chloro-2-oxo-1,3-benzo Azol-3(2H)-yl)acetic acid (38.5 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com