Growth hormone composition
A dry composition, growth hormone technology, applied in the directions of drug delivery, freeze-dried delivery, medical preparations of inactive ingredients, etc., can solve the problems of increasing the concentration of immediately available drugs, not meeting medical needs, overdosing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
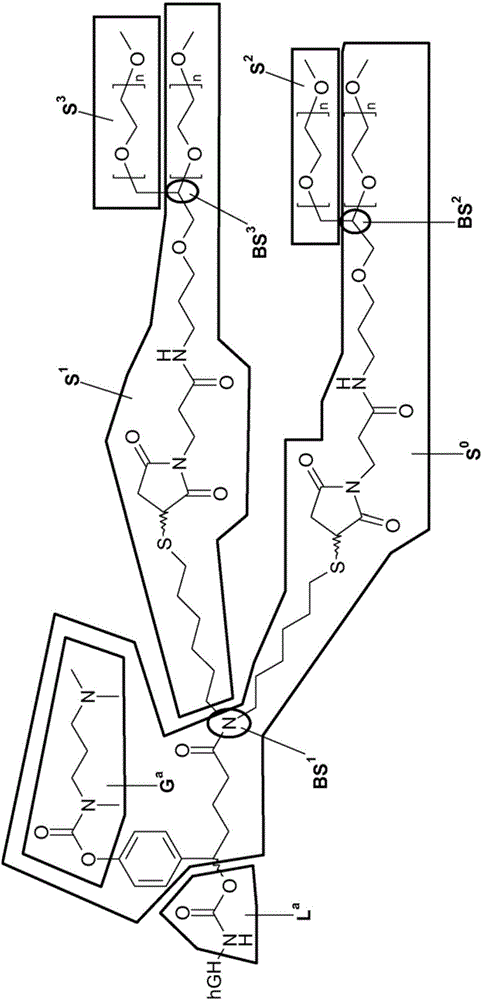
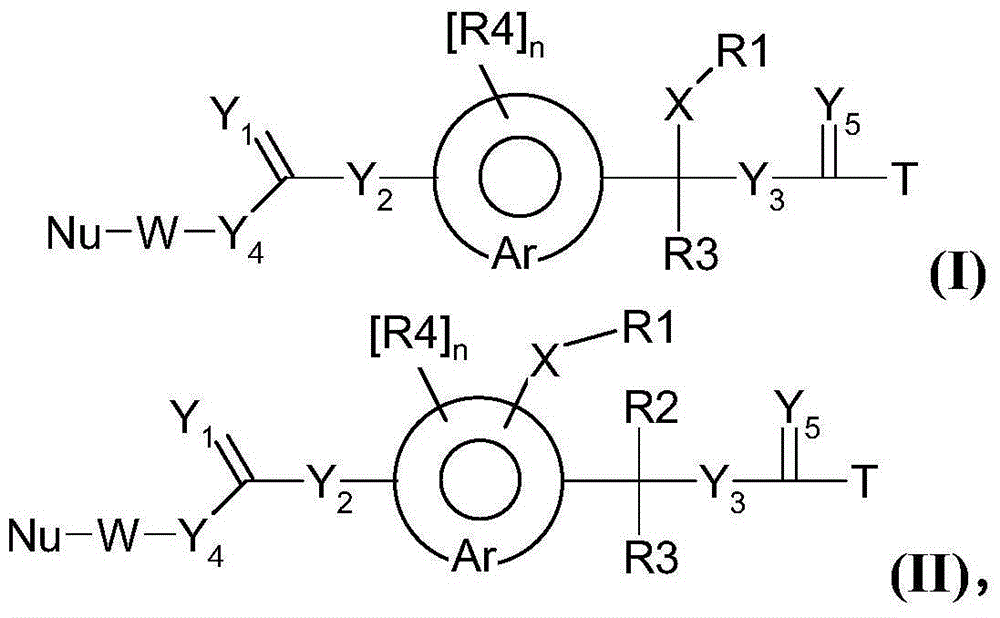
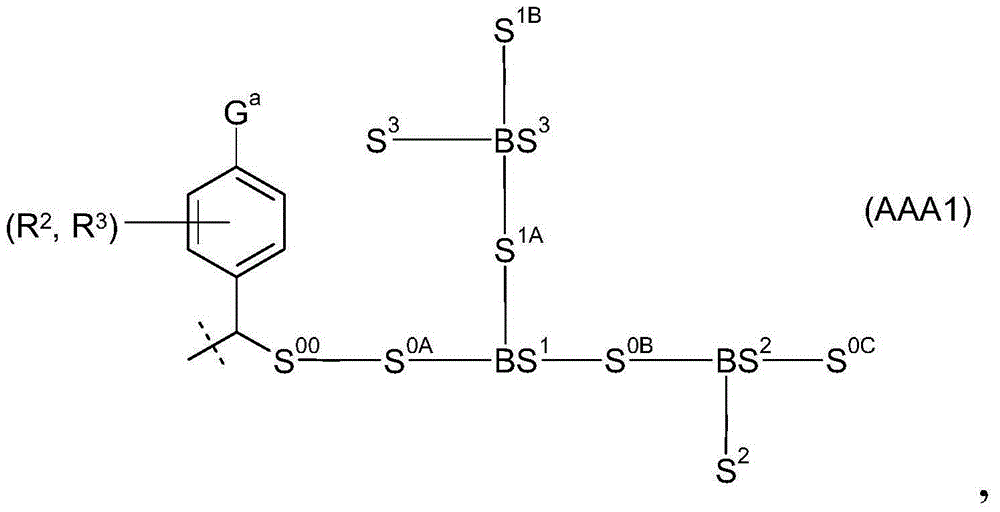
[0397] Example 1: Synthesis of hGH polymer prodrugs
[0398]
[0399] hGH polymer prodrug (1) with c~500 was synthesized as described in WO-A2009 / 133137.
Embodiment 2
[0400] Example 2: Stability Test of Compositions Containing hGH Polymer Prodrugs
[0401] Five different lyophilized compositions (Cl, C2, C3, C4 and C5) of the hGH polymer prodrug were prepared. Each composition contained an amount of hGH polymer prodrug 1 to yield a concentration of 5 mg / ml after reconstitution. Formulations were placed upright in an incubator set at 40°C / 75%RH. After 17 days, one vial of each formulation was removed from each incubator, reconstituted with sterile water for injection, and used for analysis.
[0402]
[0403]
[0404]
Embodiment 3
[0405] Example 3: Stability Test of Compositions Containing hGH Polymer Prodrugs
[0406] Three different lyophilized compositions (C6, C7 and C8) of the hGH polymer prodrug were prepared. Each composition contained an amount of hGH polymer prodrug 1 to yield a concentration of 30 mg / ml after reconstitution. Formulations were placed upright in an incubator set at 40°C / 75%RH and an incubator set at 2-8°C. At each time point, one vial of each formulation was removed from each incubator, reconstituted with sterile water for injection and analyzed.
[0407]
[0408]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com