A method for expressing antimicrobial peptide cecropin DC1 in insect cells
An insect cell and antibacterial peptide technology, applied in the field of insect cells expressing the antibacterial peptide CecropinDC1, can solve the problems of limited expression, unstable plasmid transformants, easy loss of target genes, etc., and achieve the effect of high biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Cloning of Antimicrobial Peptide Cecropin DC1 Gene
[0026] According to the needs of gene modification and cloning of the antibacterial peptide Cecropin DC1, primers were designed and synthesized using Primer premier 5.0 software:
[0027] P1: 5'ATG AAA TGG AAG TTG TTC AAA AA 3';
[0028] P2: 5’GTT AGC CAA GGC AGT AGC 3’
[0029] The PCR amplification reaction is as follows: pre-denaturation at 93°C for 5 minutes; denaturation at 93°C for 30 seconds; annealing at 58°C for 60 seconds; extension at 72°C for 1 minute; after 30 cycles, extension at 72°C for 10 minutes and incubation at 4°C.
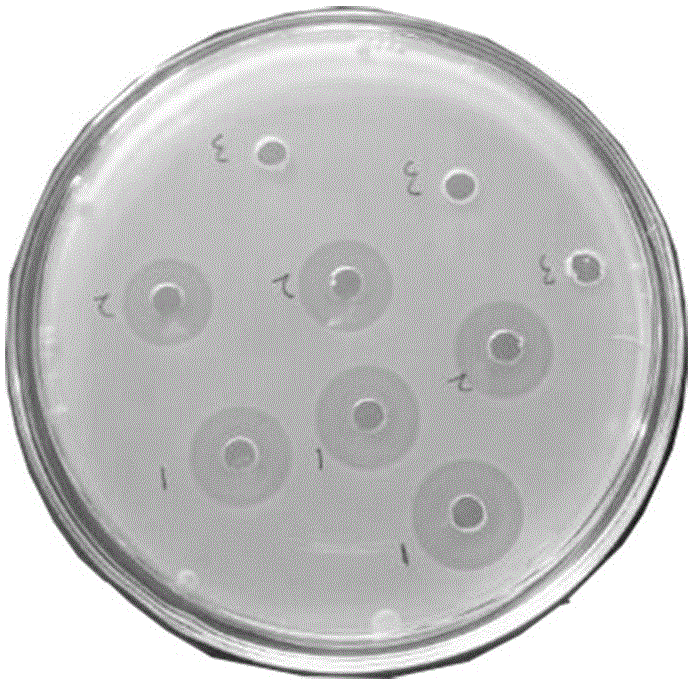
[0030] Use 1% agarose gel electrophoresis to carry out PCR product analysis to antimicrobial peptide Cecropin DC1 gene (the result is as follows figure 1 Shown) Swimming lane 1 is compared with the DNA molecular weight marker (DL 2000), and the target fragment of about 140 can be clearly seen, indicating that the antimicrobial peptide Cecropin DC1 gene was successfully synthesized. ...
Embodiment 2
[0032] get recombinant virus
[0033] The constructed recombinant transfer vector was co-transfected with Sf21 insect cells with the polynucleated polyhedrosis virus AcNPV-DNA of Spodoptera californica, and the recombinant virus expressing the antimicrobial peptide Cecropin DC1 was obtained in the following manner.
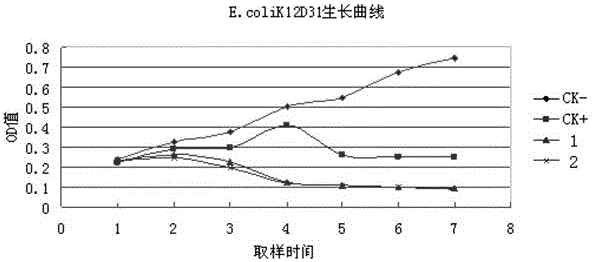
[0034] Add 2 µg recombinant transfer vector DNA, 10 µg AcNPV-DNA and 0.8 ml co-transfection buffer to a 1.5 ml sterilized Eppendorf tube, incubate the mixture for 30 min at room temperature and co-transfect Sf21 insect cells in logarithmic growth phase; Then the co-transfected Sf21 cells were washed twice with serum-free medium Sf-900 II SFM medium (without antibiotics or other additives), and then cultured at 27°C with fresh medium containing 10% FCS On the 4th to 6th day, the medium supernatant was collected as the virus stock solution to screen the recombinant virus. The recombinant virus was screened by plaque to obtain a virus solution. The virus was named as...
Embodiment 3
[0036] Extraction of recombinant baculovirus
[0037] 1) Take a 1.5 ml centrifuge tube and add 20 µl of Proteinase K solution.
[0038] 2) Add 200 µl serum or plasma to the centrifuge tube, then add 200 µl Buffer GB, and vortex for 15 sec.
[0039] 3) Incubate at 56°C for 15 minutes, centrifuge briefly, and collect the solution on the tube wall to the bottom of the tube.
[0040] 4) Add 250 µl of absolute ethanol, vortex for 15 sec, place at room temperature for 5 min, centrifuge briefly, and collect the solution on the tube wall to the bottom of the tube.
[0041] 5) Put a Spin Columns CG * Put it into Collection Tubes (2 ml), transfer the solution obtained in the previous step to a centrifugal adsorption column, centrifuge at 10 000 rpm for 30 sec, and discard the waste liquid in the collection tube.
[0042] 6) Add 500 µl of Buffer GD to the adsorption column, centrifuge at 10 000 rpm for 30 sec at room temperature, and discard the waste liquid in the collection tube.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com