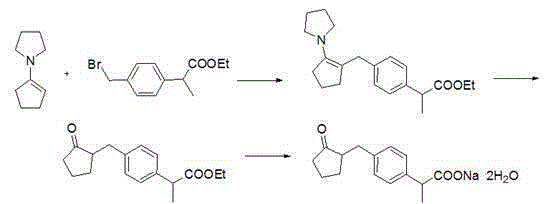
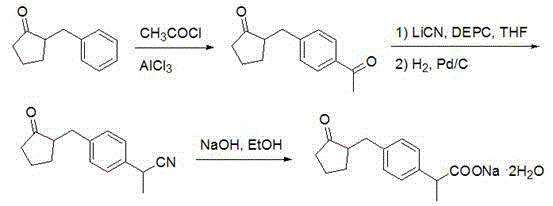
Method for synthesizing loxoprofen sodium
The technology of a kind of loxoprofen sodium, synthetic method is applied in the synthetic field of loxoprofen sodium, can solve problems such as low product conversion rate, sensitive reaction conditions, complex reaction route, achieves high product conversion rate, simple and convenient operation, The effect of a simple reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
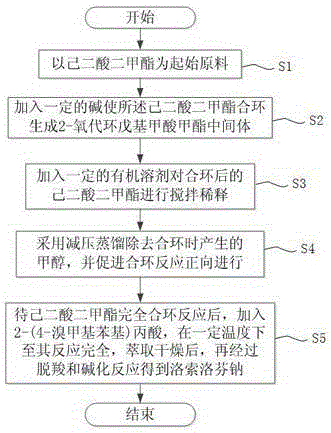
[0024] See figure 1 , the synthetic method of loxoprofen sodium provided by the invention, comprises the steps:
[0025] Step S1: using dimethyl adipate as a starting material;
[0026] Step S2: adding a certain base to make the dimethyl adipate ring-closed to generate methyl 2-oxocyclopentylcarboxylate intermediate; such as organic base, inorganic base or alkali metal compound;
[0027] Step S3: add a certain amount of organic solvent to stir and dilute the dimethyl adipate after ring closure; the mass ratio of base to dimethyl adipate and organic solvent is preferably 0.5:1:1 to 3:1:10 Between; the selection of organic solvent is comparatively important, neither can be removed during vacuum distillation because of its lower boiling point, nor can it be difficult to recover and separate when its boiling point is higher in post-treatment. Recovery cost; after many experiments, the organic solvent is preferably MeOH (methanol), EtOH (ethanol), EtOH 2 O (diethyl ether), MTBE ...
Embodiment 1
[0032] 1000mL four-necked glass bottle, equipped with internal temperature thermometer, condenser and mechanical stirring, put 130g dimethyl adipate at room temperature, add toluene 450mL under stirring, mix well, add metal sodium 17.2g in batches under stirring, heat up and reduce The methanol produced in the reaction was removed by distillation under pressure. After the reaction of dimethyl adipate was complete, 121 g of 2-(4-bromomethylphenyl) was added, and the temperature was raised until the reaction was complete. After cooling down to room temperature, 300 mL of water was added, and 20 mL of Dilute hydrochloric acid (6MHCl) with a concentration of 6 moles per liter adjusted the system to about PH = 6-7, extracted with toluene, dried and concentrated to dryness to obtain 191g of yellow oil, which is the precursor of loxoprofen sodium, without purification Directly put into the next step of decarboxylation reaction.
Embodiment 2
[0034] 1000mL four-necked glass bottle, equipped with internal temperature thermometer, condenser and mechanical stirring, put 130g dimethyl adipate at room temperature, add toluene 200mL under stirring, mix well, add metal sodium 17.2g in batches under stirring, heat up and reduce Remove the methanol generated in the reaction by distillation under pressure. After the reaction of dimethyl adipate is complete, add 121 g of 2-(4-bromomethylphenyl), heat up and react until the reaction is complete, cool down to room temperature, add 300 mL of water, and add 20 mL of 6M HCl Adjust the system to PH=6-7, extract with toluene, dry and concentrate to dryness to obtain 182 g of yellow oil, which is directly put into the next reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com