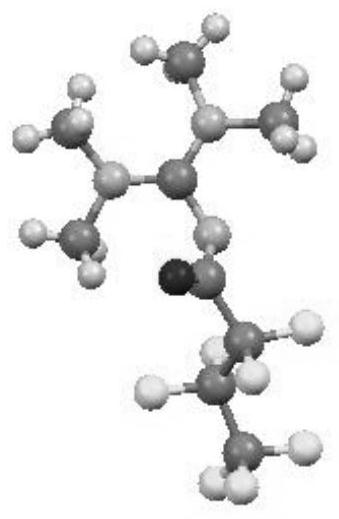
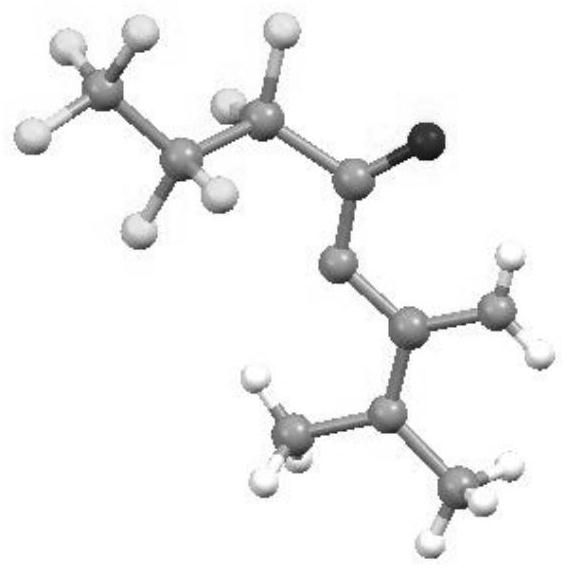
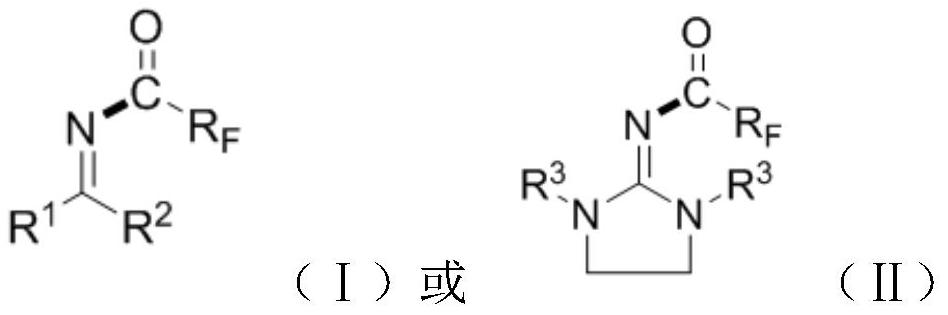
a n 2 - Polyfluoroalkyl acyl guanidine compound and preparation method thereof
A technology of polyfluoroalkylacylguanidine and guanidine compounds, which is applied in the field of preparation of N2-polyfluoroalkylacylguanidine compounds, and achieves the effects of novel structure, cheap and easy-to-obtain raw materials, and specific stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Nitrogen-(bis(dimethylamino)methylene)-2,2,3,3,4,4,4-heptafluorobutanamide
[0033] 1. At room temperature, tetramethylguanidine (1.0 mmol) was put into a 25 mL round bottom flask, and 2 mL of acetonitrile (water content 0.1%) solvent was injected. Then, perfluoroiodobutane (1.1 eq) was added. Under the irradiation of 36W CFL lamp, the reaction was monitored by TLC until the reaction was complete (about 10h). The resulting reaction product was poured into 100 mL of water, extracted three times with dichloromethane, the organic phases were combined, dried, and the organic solvent was distilled off under reduced pressure to obtain a crude product.
[0034] 2. the crude product is carried out to silica gel column chromatography (silica gel needs to use Et 3 N / PE=1:9 immersion overnight pretreatment), eluting with eluent (petroleum ether: ethyl acetate=1:1 (V:V)), collecting the eluate, and distilling under reduced pressure to obtain a white solid , which is th...
Embodiment 2
[0041] Example 2 (E)-nitrogen-(amino(dimethylamino)methylene)-2,2,3,3,4,4,4-heptafluorobutanamide
[0042] 1. At room temperature, put 1,1-dimethylguanidine hydrochloride (1.0mmol) and sodium hydroxide (1.0eq) into a 25mL round bottom flask, inject 2mL of acetonitrile (water content 0.1%) solvent, and neutralize for 4h The acid was removed, then perfluoroiodobutane (1.1 eq) was added. The reaction was performed under 36W CFL light irradiation, and monitored by TLC until the reaction was complete (about 12h). The resulting reaction product was poured into 100 mL of water, extracted three times with dichloromethane, the organic phases were combined, dried, and the organic solvent was distilled off under reduced pressure to obtain a crude product.
[0043] 2. the crude product is carried out to silica gel column chromatography (silica gel needs to use Et 3 N / PE=1:9 immersion overnight pretreatment), eluting with eluent (petroleum ether: ethyl acetate=1:1 (V:V)), collecting the ...
Embodiment 3
[0050] Example 3 Nitrogen-(diaminomethylene)-2,2,3,3,4,4,4-heptafluorobutanamide
[0051] 1. At room temperature, put guanidine hydrochloride (1.0mmol) and sodium hydroxide (1.0eq) into a 25mL round bottom flask, inject 2mL of acetonitrile (water content 0.1%) solvent, neutralize for 4h to remove acid, and then add all Fluoroiodobutane (1.1 eq). The reaction was performed under 36W CFL light irradiation, and monitored by TLC until the reaction was complete (about 14h). The resulting reaction product was poured into 100 mL of water, extracted three times with dichloromethane, the organic phases were combined, dried, and the organic solvent was distilled off under reduced pressure to obtain a crude product.
[0052] 2. the crude product is carried out to silica gel column chromatography (silica gel needs to use Et 3 N / PE=1:9 immersion overnight pretreatment), eluting with eluent (petroleum ether: ethyl acetate=1:1 (V:V)), collecting the eluate, and distilling under reduced pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com