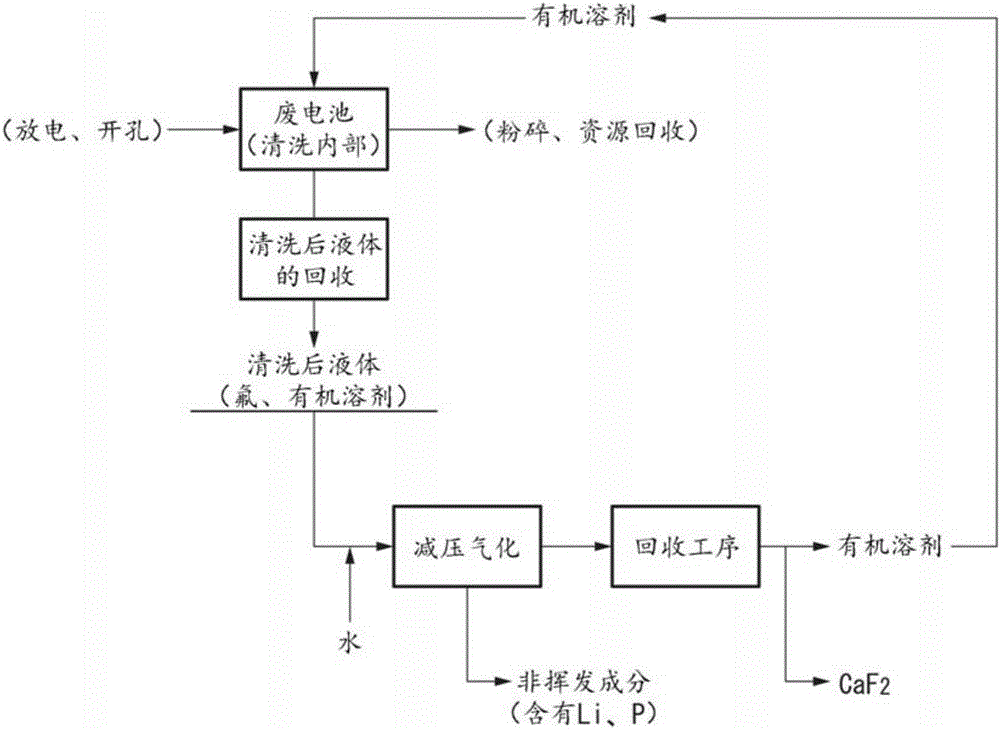
Method for processing fluorine-containing electrolyte solution
A treatment method and electrolyte technology, applied in organic electrolytes, non-aqueous electrolytes, circuits, etc., can solve problems such as difficult to reuse, and achieve the effect of safe use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] [Example 1: Cleaning]
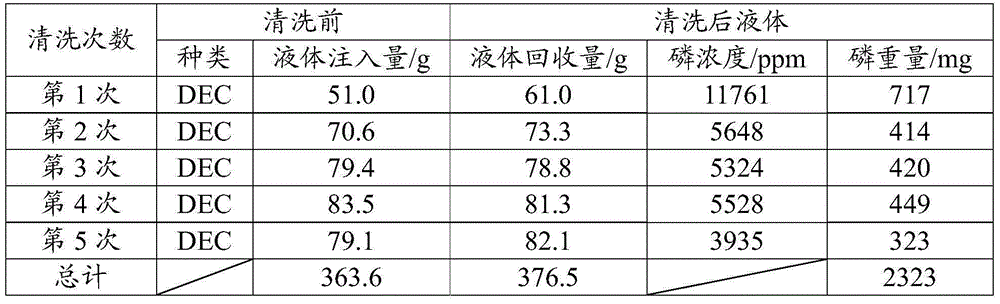
[0056] Diethyl carbonate (DEC) was used as an organic solvent for the cleaning solution, and it was injected into a used lithium-ion battery weighing 1.67 kg, and the internal pressure was reduced to 20 kPa, followed by degassing treatment, followed by ultrasonic irradiation for 3 minutes . Extraction is performed by sucking out the cleaned liquid from inside the battery. The operation from injecting the organic solvent to extracting the washed liquid was repeated five times. Table 1 shows the number of times of washing, the amount of injected liquid of the organic solvent, the amount of recovered washed liquid, and the phosphorus concentration (phosphorus weight) contained in the washed liquid. Fluorine and phosphorus in the electrolyte form PF 6 - Since the complex compound cannot measure the concentration of fluorine with a fluoride ion electrode in this form, phosphorus was used instead of fluorine as an indicator to show the cleaning eff...
Embodiment 2
[0060] [Example 2: gasification and recovery]
[0061] Pour 373g of the cleaned liquid extracted in Example 1 into an airtight container, and add 20g of water, and carry out decompression for 2 hours under the condition that the temperature of the oil bath is 120°C and the pressure is 20kPa, so that the liquid contained in the cleaned liquid Vaporization of organic solvents and hydrogen fluoride.
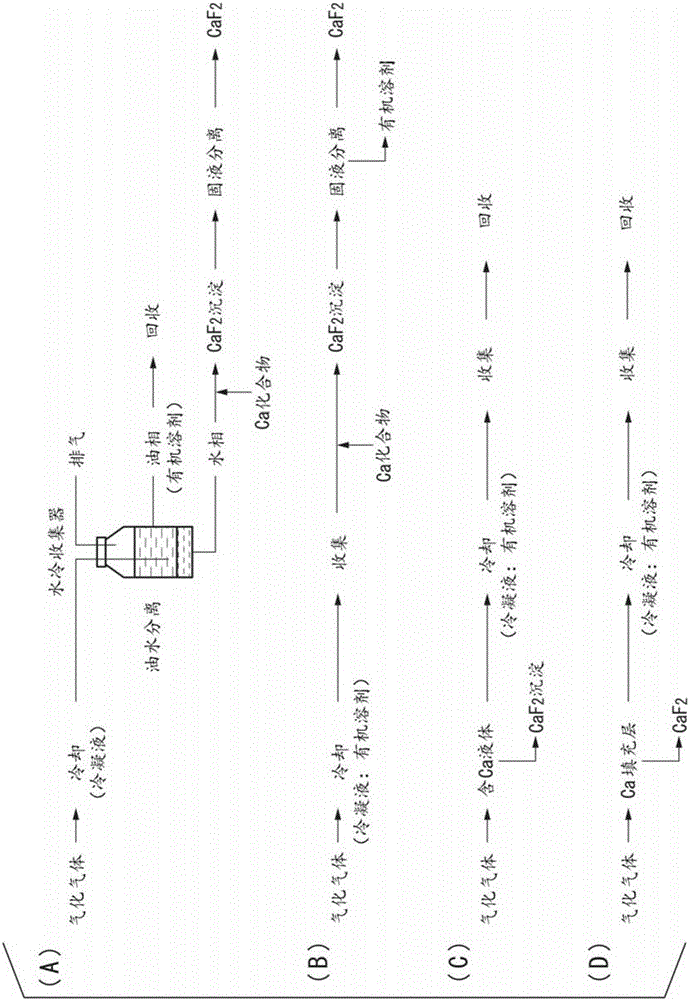
[0062] according to figure 2 In the recovery process shown in (B), this vaporized gas is introduced into a cooler, cooled and condensed to 2° C., and 325 mL of condensate is recovered. The fluorine concentration of the condensate was 14400 mg / L, and the pH was 1.0. 13.0 g of calcium carbonate was added to the condensate to form a precipitate. It was confirmed by powder X-ray diffraction that the precipitate subjected to solid-liquid separation was calcium fluoride. The recovered amount of calcium fluoride was 10.3 g (the recovered amount of fluorine was 4.59 g), and the purity ...
Embodiment 3
[0063] [Example 3: Reuse of DEC]
[0064] Except purifying the mixed solvent mainly composed of diethyl carbonate (DEC) recovered in Example 2 to be used as the organic solvent of the cleaning liquid, the recovered organic solvent was used in the same manner as in Example 1 to treat The inside of the spent Li-ion battery is cleaned and the cleaned fluid is extracted from the battery. Wash and recover 5 times, the amount of each injected liquid is 50g ~ 80g, the total amount of injected liquid is 355.2g, the total amount of recovered liquid (liquid volume after cleaning) is 367.3g, and the total amount of phosphorus contained in the cleaned liquid is 2.3 g.
[0065] 20 g of water was added to the extracted cleaned liquid, and calcium fluoride and an organic solvent were recovered in the same manner as in Example 2. The recovered amount of calcium fluoride was 10.2 g (the recovered amount of fluorine was 4.54 g), and the purity was 90.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com