A kind of solid dispersion of alisartan medoxomil and the pharmaceutical composition containing the solid dispersion
一种阿利沙坦酯、固体分散体的技术,应用在药物制剂领域,能够解决老化、溶出性能下降等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
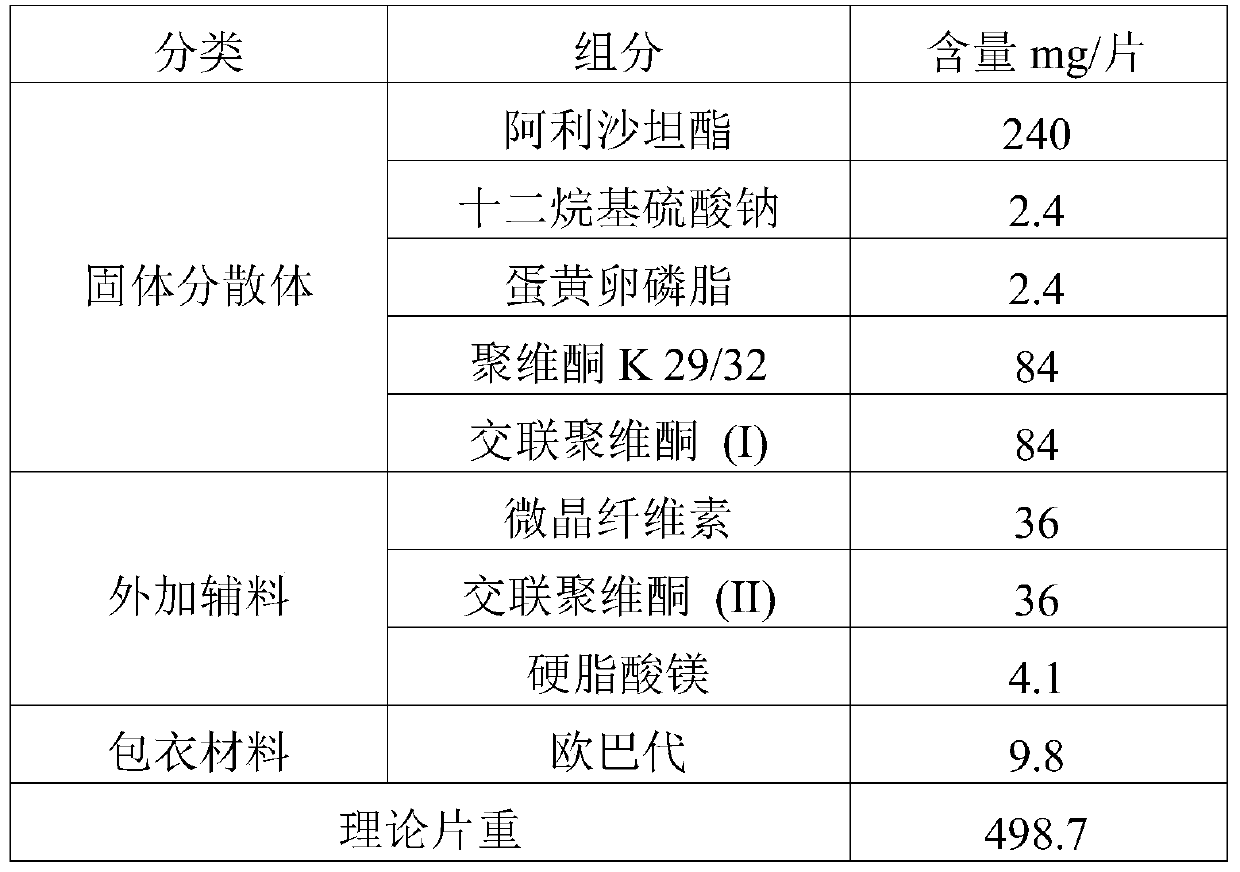
[0055] prescription:
[0056]
[0057] preparation:
[0058] 1. Preparation of solid dispersion
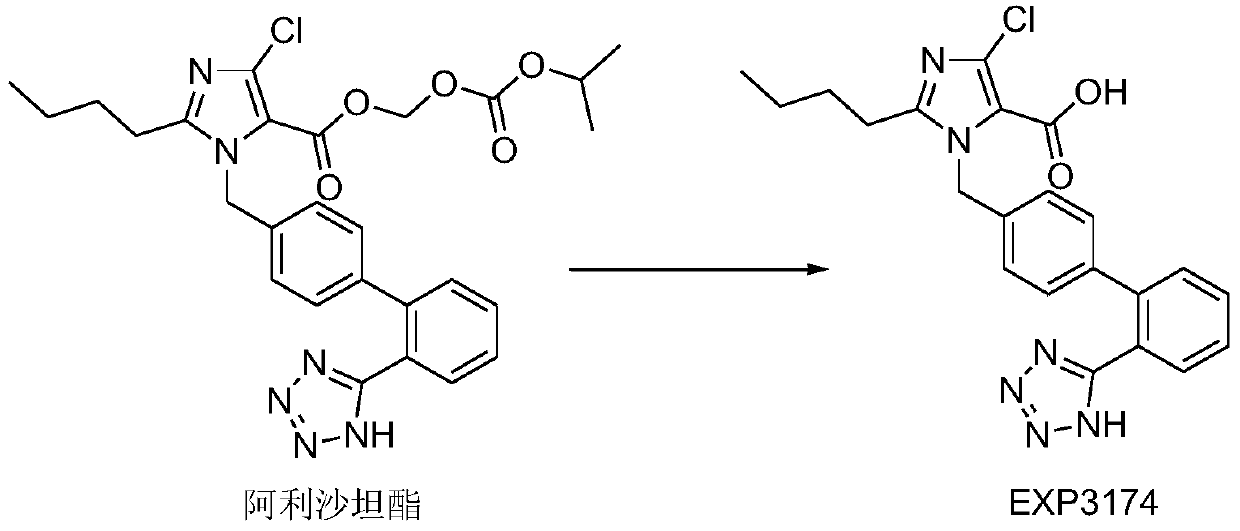
[0059] Dissolve the drug and povidone K 29 / 32 in an appropriate amount of dichloromethane-ethanol mixed solution, then add the aqueous solution of sodium lauryl sulfate and egg yolk lecithin, mix well, and set aside. Add crospovidone (I) into the fluidized bed, spray the prepared solution into the fluidized bed with a spray gun to granulate in the top spray mode, and dry to obtain the solid dispersion of alisartan medoxomil; further XRD experiment detection found that Alisartan The active ingredient of tannyl ester is highly dispersed in the solid dispersion, which proves that the preparation effect of the solid dispersion is as expected.
[0060] 2. Preparation of pharmaceutical composition
[0061] The solid dispersion is uniformly mixed with additional auxiliary materials, compressed into tablets to obtain plain tablets, and film-coated to obtain the alisartan medoxomil p...
Embodiment 2
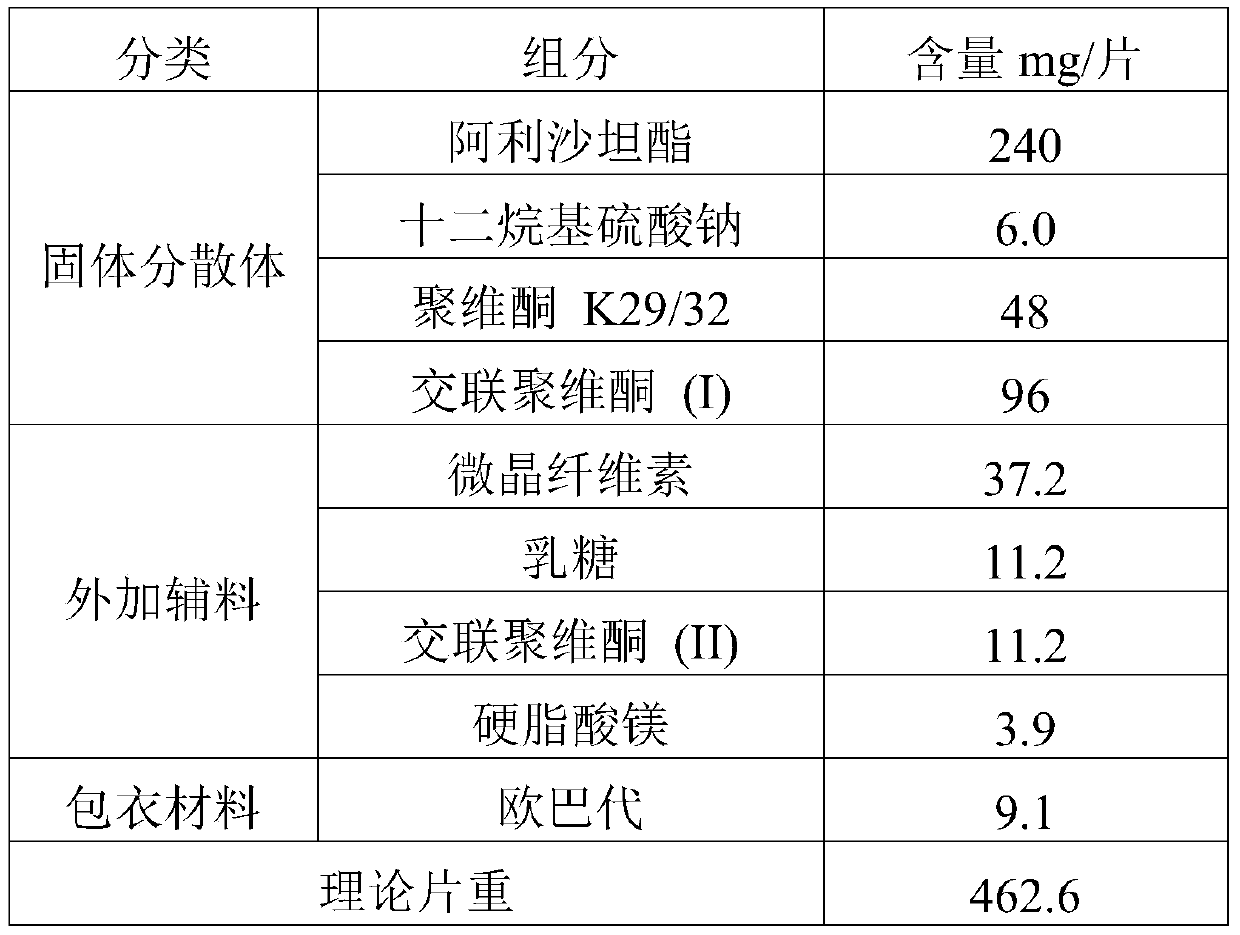
[0063] prescription:
[0064]
[0065] preparation:
[0066] 1. Preparation of solid dispersion
[0067] Dissolve the drug and povidone K29 / 32 in an appropriate amount of dichloromethane-ethanol mixed solution, then add an aqueous solution of sodium lauryl sulfate, mix well, and set aside. Add crospovidone (I) into the fluidized bed, spray the prepared solution into the fluidized bed with a spray gun to granulate in the top spray mode, and dry to obtain the solid dispersion of alisartan medoxomil; further XRD experiment detection found that Alisartan The active ingredient of tannyl ester is highly dispersed in the solid dispersion, which proves that the preparation effect of the solid dispersion is as expected.
[0068] 2. Preparation of pharmaceutical composition
[0069] The solid dispersion is uniformly mixed with additional auxiliary materials, compressed into tablets to obtain plain tablets, and film-coated to obtain the alisartan medoxomil pharmaceutical compositio...
Embodiment 3
[0071] prescription:
[0072]
[0073] preparation:
[0074] 1. Preparation of solid dispersion
[0075] Dissolve the drug and povidone K29 / 32 in an appropriate amount of dichloromethane-ethanol mixed solution, then add egg yolk lecithin, dissolve and mix evenly, and set aside. Microcrystalline cellulose and crospovidone (I) are added in the fluidized bed, and the prepared solution is sprayed into the fluidized bed with a spray gun to granulate in the top spray mode, and dried to obtain alisartan medoxomil solid dispersion; further XRD Experimental testing found that the active ingredient of alisartan medoxomil was highly dispersed in the solid dispersion, which proved that the preparation effect of the solid dispersion reached expectations.
[0076] 2. Preparation of pharmaceutical composition
[0077] The solid dispersion is uniformly mixed with additional auxiliary materials, compressed into tablets to obtain plain tablets, and film-coated to obtain the alisartan medo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com