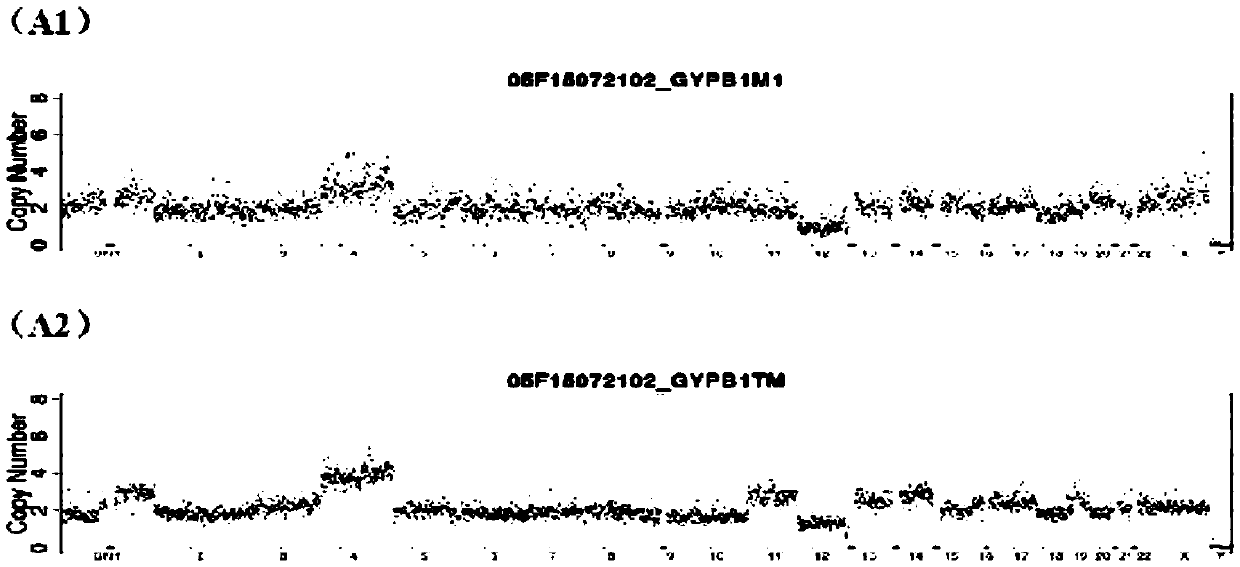
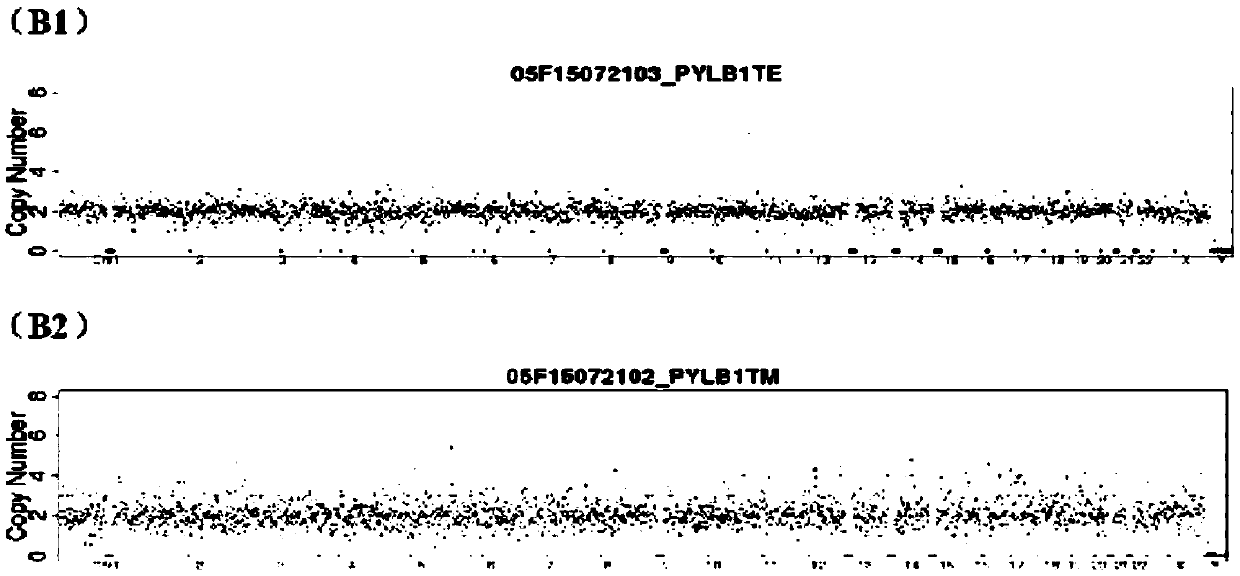
Method for detecting embryo chromosome abnormality by utilizing blastula culture solution
A chromosomal abnormality and culture medium technology, applied in the fields of biomedicine and molecular cell biology, can solve the problems of lifelong health to be observed, faulty test results, termination of embryonic development, etc., to avoid cell loss and damage, and simplify the operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Select two in vitro fertilized embryo samples, A and B, and use blastocyst cell detection method and blastocyst culture fluid detection method to evaluate their chromosomal status respectively. The specific steps are as follows:
[0053] 1. Acquisition of Blastocyst Culture Medium
[0054] 1) After the fertilized eggs obtained by single sperm injection are cultured to the blastomere stage on the third day, the embryos are transferred to the newly prepared blastocyst culture microdrops for blastocyst culture.
[0055] 2) Aspirate the blastocyst-forming embryos and transfer them to a new blastocyst culture medium / or enter the vitrification process. The remaining original blastocyst culture medium (about 30ul) is sample A and sample B that need to be collected for PGS .
[0056] 2. Collection of blastocyst culture medium
[0057] 1) Place the collection tube containing 10 microliters of lysate (Tris-Cl40mM with a pH of 7.2, EDTA1mM, KCl15mM and 3% TritonX-100) at room te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com