Method for isolating tumor cell-derived exosomes from urine
A tumor cell and exosome technology, applied in the biological field, can solve the problems of difficult clinical application promotion, unsatisfactory, cumbersome operation, etc., and achieve the effects of large clinical application value, easy sampling and non-invasive, and easy to obtain in large quantities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Extraction of exosome derived from tumor cells in the urine of patients with renal clear cell carcinoma
[0053] ①With the patient’s informed and ethical consent, collect 25ml of preoperative urine specimens from tumor patients (pathological biopsy has confirmed renal clear cell carcinoma) in a 50ml centrifuge tube, and centrifuge at 4°C and 300g for 10 minutes , separated to remove the cells in the urine, and then get the supernatant.
[0054] ② Freeze-dry the upper liquid obtained in ① to obtain a precipitate. Wherein, freeze-drying is completed in a freeze-drying apparatus, specifically: after freezing the upper layer liquid obtained in ① at -80° C., freeze-drying overnight (6 to 16 hours) on a freeze-drying apparatus at -40 to -50° C.
[0055] ③ Resuspend the lyophilized precipitate with 1ml of 1×PBS to obtain the first resuspension.
[0056] ④ Centrifuge the first resuspension at 4°C and 10,000 g for 30 minutes, separate to remove organelles and other ...
Embodiment 2
[0086] Example 2: Extraction of tumor-derived exosomes in the urine of patients with esophageal cancer
[0087] The tumor-derived exosome in the urine of patients with esophageal cancer was extracted by basically the same method as in Example 1. That is: ① The urine sample in ① is a 50ml urine sample from a patient with esophageal cancer before operation, and other steps are exactly the same as in Example 1.
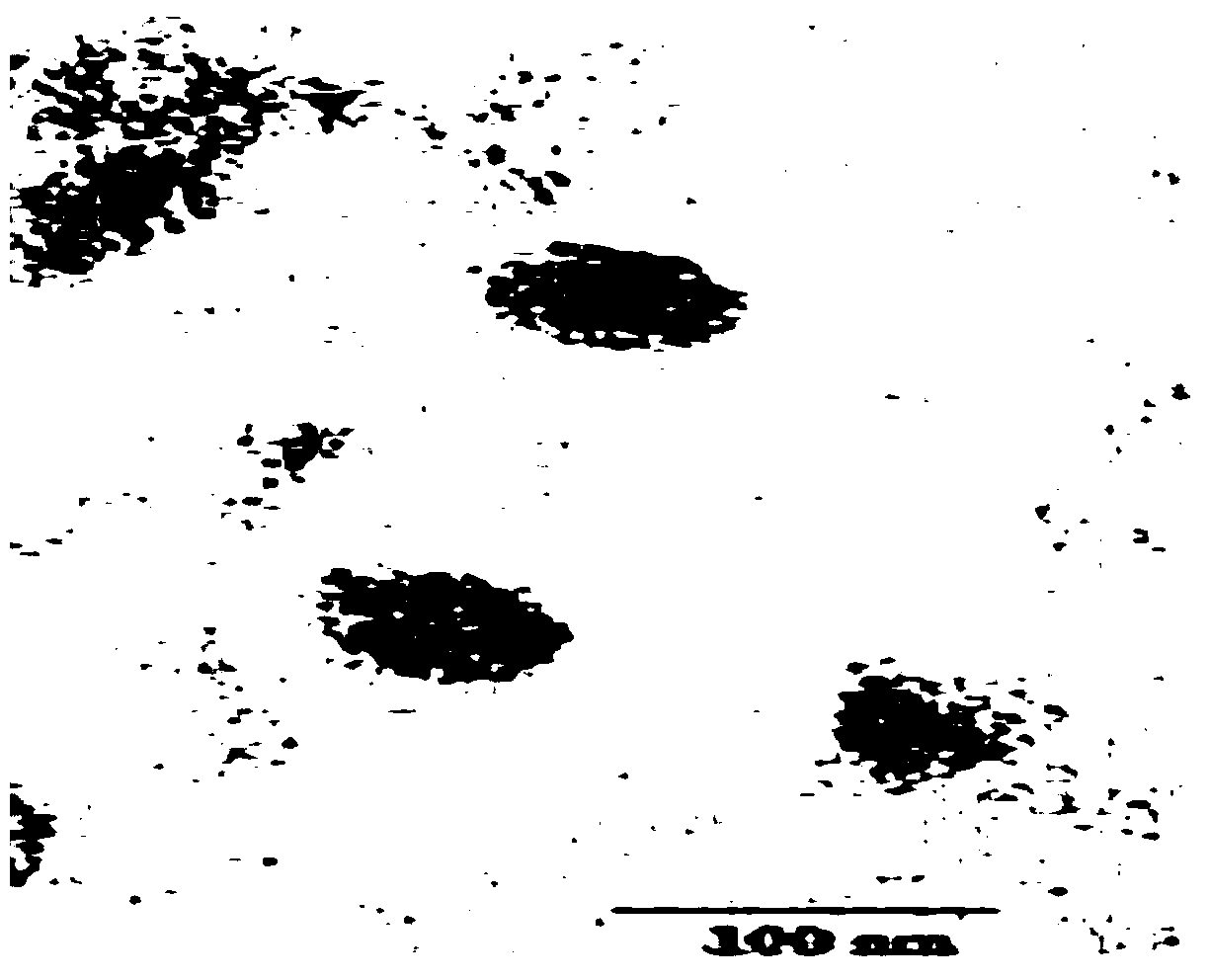
[0088] Similarly, the method in Example 1 was used to observe the product obtained in Example 2 with an electron microscope, and the morphology of exosomes consistent with the literature was also observed.
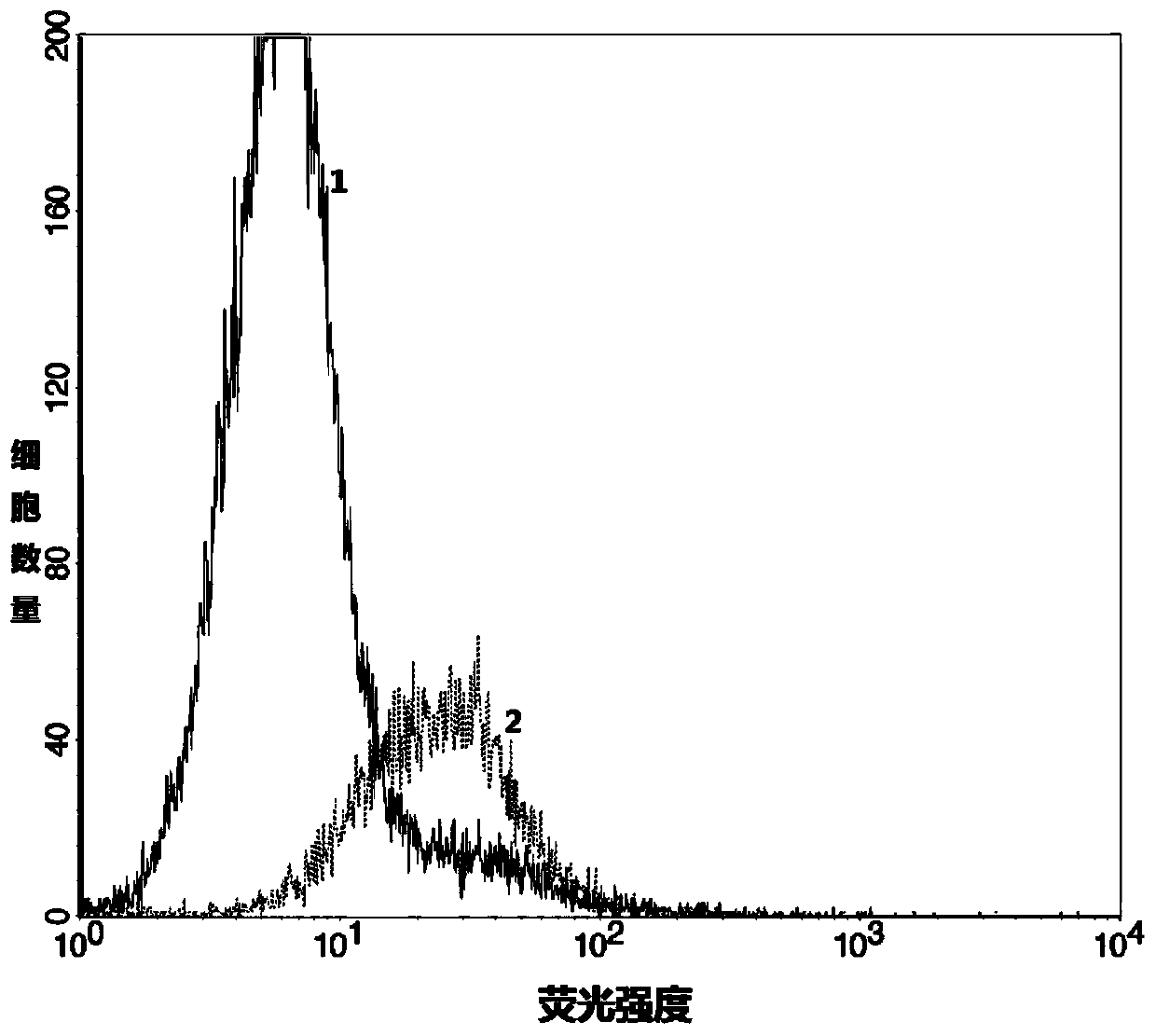
[0089] Further, the expression of CD63 and CD9 was detected by flow cytometry:
[0090] Resuspend the product obtained in Example 2 (i.e., magnetic bead-bound tumor cell-derived exosome) with 1 ml of 1×PBS to obtain 1 ml of esophageal cancer patient urine tumor-derived exosome resuspension, and resuspend the Add 5ul of flow cytometry antibodies CD63 and CD9 to the ...
Embodiment 3
[0093] Example 3: Extraction of tumor-derived exosomes in the urine of lung cancer patients
[0094] The tumor-derived exosome in 25 ml of urine of a patient with lung cancer was extracted by basically the same method as in Example 1. That is: ① The urine sample in the middle is the urine sample of the patient with lung cancer before operation, and other steps are exactly the same as in Example 1.
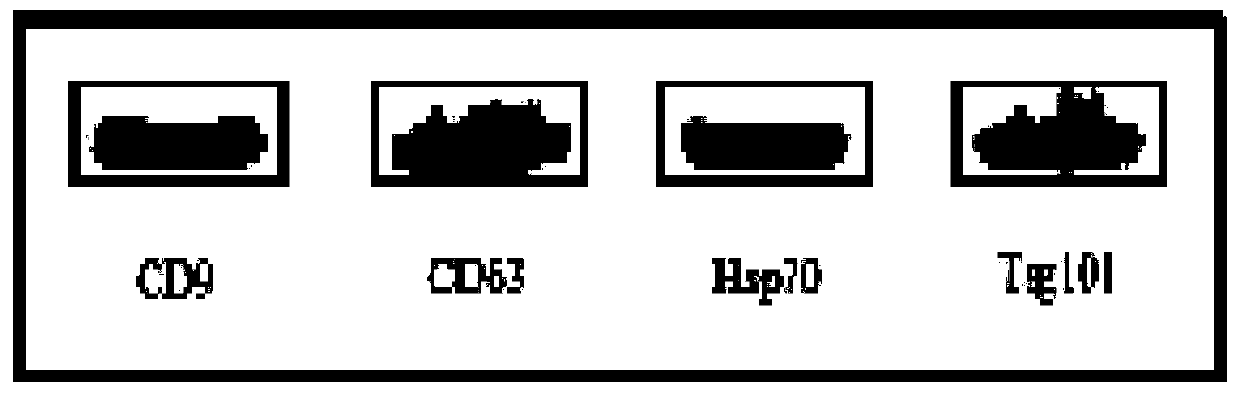
[0095] Using the product characterization method in Example 1, the product obtained in Example 3 was observed and tested. The results showed that: the product obtained in Example 3 had an exosome morphology that was consistent with that recorded in the literature, and the unique characteristics of exosomes were detected in the product. The expression of proteins CD9, CD63, Hsp70, Tsg101 and the strong expression of tumor antigen EpCAM protein prove that the product obtained in Example 3 is the exosome derived from tumor cells extracted from urine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com