Polyamide-imide resin, and curable resin composition and cured product of same
一种聚酰胺酰亚胺树脂、固化性树脂的技术,应用在固化性树脂组合物及其固化物,聚酰胺酰亚胺树脂领域,能够解决可使用时间短、处理性不充分等问题,达到可使用时间长、优异透明性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0129] Next, the present invention will be further described in detail by showing examples. In the examples, "parts" and "%" are based on mass unless otherwise specified.
Synthetic example 1
[0131] [Preparation of polyamideimide resin (A1-1)]
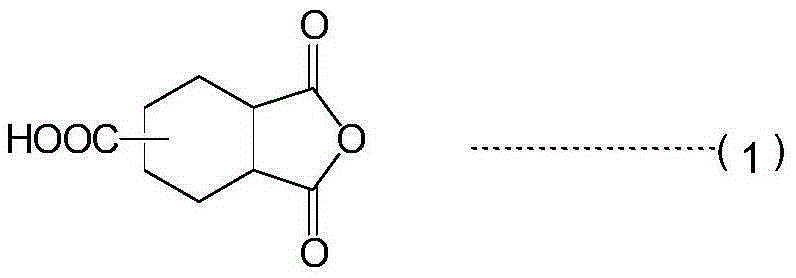
[0132] Add PGMAc (propylene glycol monomethyl ether acetate) 1086g, IPDI3N (isocyanurate type triisocyanate synthesized by isophorone diisocyanate: NCO%=17.2 ) 587.3g (0.80mol) and cyclohexane-1,3,4-tricarboxylic acid-3,4-anhydride 499.1g (2.52mol), the temperature was raised to 140°C. The reaction proceeds with foaming. The reaction was carried out at this temperature for 8 hours. A pale yellow liquid was formed in the system, and the characteristic absorption was measured by infrared spectroscopy. As a result, the characteristic absorption of the isocyanate group was 2270cm -1 Completely disappeared at 1780cm -1 、1720cm -1 Absorption of imide groups was confirmed. The acid value was 212 KOHmg / g in terms of solid content, and the molecular weight was 4,700 in number average molecular weight in terms of polystyrene. The concentration of acid anhydride groups was 1.14 mmol / g in terms of solid content. In addition, the...
Synthetic example 2
[0136] [Preparation of polyamideimide resin (A1-2)]
[0137] Add EDGA (diethylene glycol monoethyl ether acetate) 4628g, IPDI3N (isocyanurate type triisocyanate synthesized by isophorone diisocyanate: NCO%= 17.2) 2070 g (2.83 mol) and 1386 g (7 mol) of cyclohexane-1,3,4-tricarboxylic acid-3,4-anhydride, heated to 140°C. The reaction proceeds with foaming. The reaction was carried out at this temperature for 8 hours. A pale yellow liquid was formed in the system, and the characteristic absorption was measured by infrared spectroscopy. As a result, the characteristic absorption of the isocyanate group was 2270cm -1 Completely disappeared at 1780cm -1 、1720cm -1 Absorption of imide groups was confirmed. The acid value was 140 KOHmg / g in terms of solid content, and the molecular weight was 5800 in number average molecular weight in terms of polystyrene. The concentration of the acid anhydride group was 0.75 mmol / g in terms of solid content. In addition, the concentration of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com