Stable human growth hormone liquid preparation
A technology of human growth hormone and liquid preparation, applied in the field of stable liquid preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation and Analysis of Human Growth Hormone Liquid Preparation
[0032] The liquid formulations of the present invention are prepared as follows. 4.0 mg / ml of natural human growth hormone (Daewoong, Korea, which was supplied as a bulk solution before being lyophilized) was mixed with sodium acetate (Sigma), sodium citrate as a buffer to adjust the pH to the range of 6.0-6.2 (Sigma) or a 10 mM aqueous solution of sodium dihydrogen phosphate (Sigma). After the solution was adjusted to the final concentration, it was mixed with excipient (D-mannitol, Sigma), surfactant {polyethylene glycol-15 polyhydroxystearate (SolutolHS15, BASF), poloxamer 188 (Lutrol F68, BASF), Poloxamer 407 (Lutrol F127, BASF), polyoxyethylene ether castor oil ELP (BASF), Tween20 (CRILLET1HP, Croda) or Tween80 (CRILLET4HP, Croda)}, stabilizer (polyethylene glycol Alcohol 300 (PEG300, BASF), polyethylene glycol 400 (PEG400, BASF), PVPK-12 (Kollidon12PF, BASF), PVPK-15 (Kollidon15PF, ...
Embodiment 2
[0034] Example 2: Evaluation of the Effect of Surfactants on the Stability of Human Growth Hormone
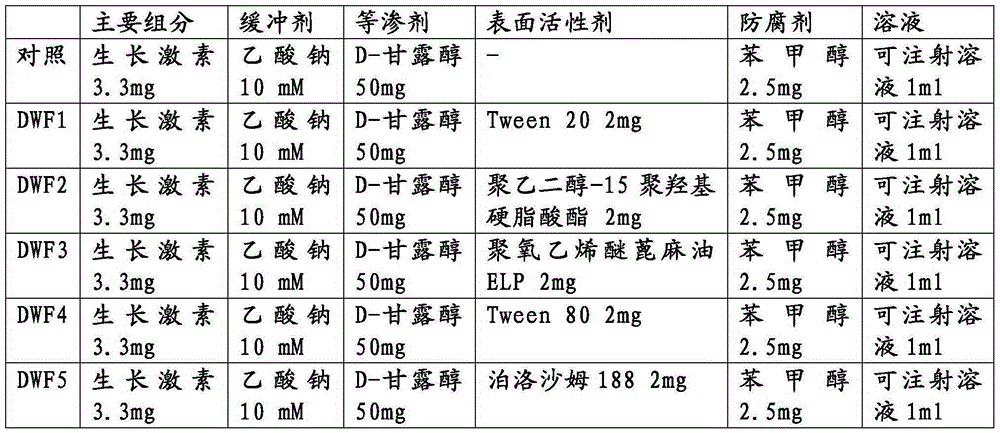
[0035] In order to study the influence of pharmaceutically acceptable surfactants on the stability of human growth hormone, human growth hormone liquid preparations were prepared using the components listed in Table 1, and then analyzed according to the same method as in Example 1.
[0036] Table 1
[0037]
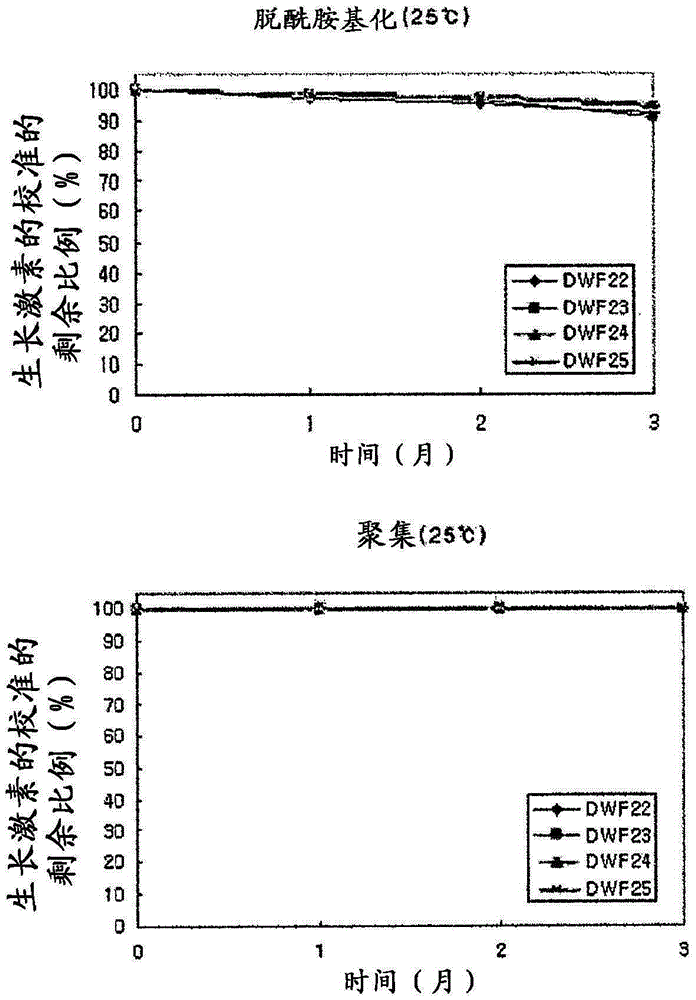
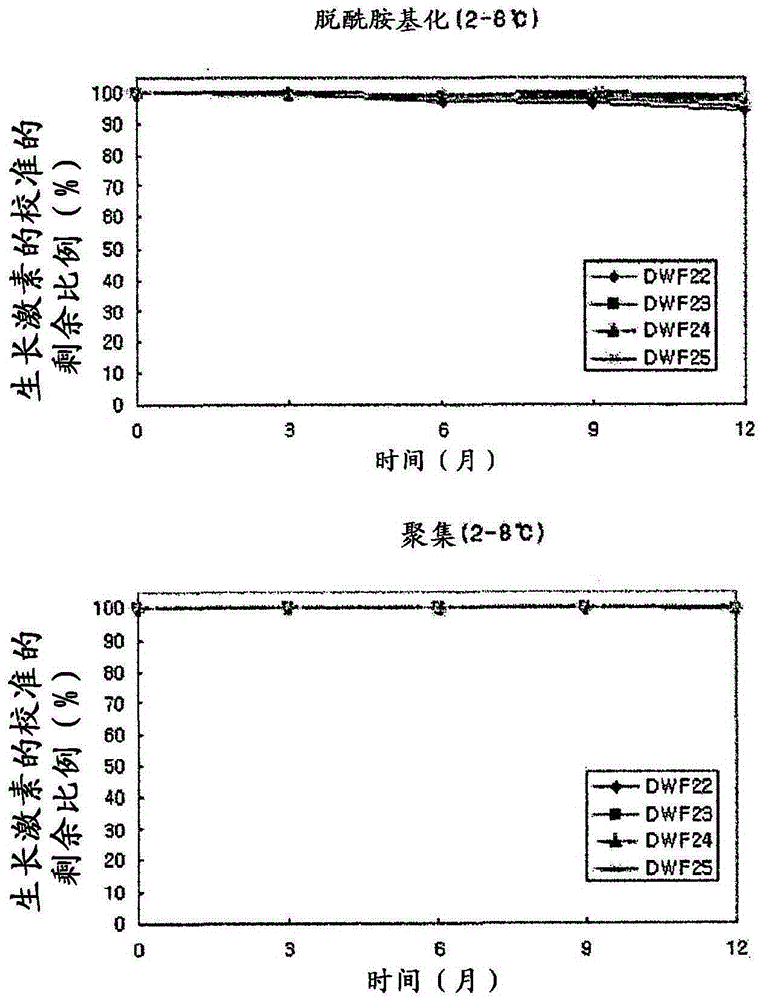
[0038] The thus-prepared liquid preparations were evaluated for deamidation and aggregation of human growth hormone according to the type of surfactant, and the results are given in Table 2 below.
[0039] In Table 2, deamidation (%) indicates the calibrated remaining proportion (%) of undeamidated active human growth hormone according to the time at 40°C, and aggregation indicates activity that does not form dimers or aggregates Calibrated remaining proportion (%) of human growth hormone.
[0040] Table 2
[0041]
[0042]
[0043] Preparation of formulations ...
Embodiment 3
[0044] Example 3: Evaluation of the Effect of Stabilizers on the Stability of Human Growth Hormone
[0045] In order to study the influence of different stabilizers on the stability of human growth hormone, liquid preparations of human growth hormone were prepared using the components listed in Table 3, and then analyzed according to the same method as in Example 1.
[0046] table 3
[0047]
[0048] The deamidation and aggregation of the liquid formulations thus prepared were evaluated according to the type of stabilizer, and the results are given in Table 4 below.
[0049] Table 4
[0050]
[0051]
[0052] As shown in Table 4, among the stabilizers used, PEG300 (polyethylene glycol 300) and two amino acids (L-Lys·HCl and L-Arg·HCl) were found to stabilize human growth hormone. In particular, when L-lysine and L-arginine were used, aggregation in the formulation was inhibited to a higher degree.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com