Benzo(c)acridine derivative, and preparation method and use thereof
A derivative, acridine technology, used in the field of anti-tumor drugs and medicine, can solve the problems of enhanced anti-cancer activity and weak anti-cancer activity, and achieve the effects of increased purity, strong anti-tumor activity, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0025] Example 1
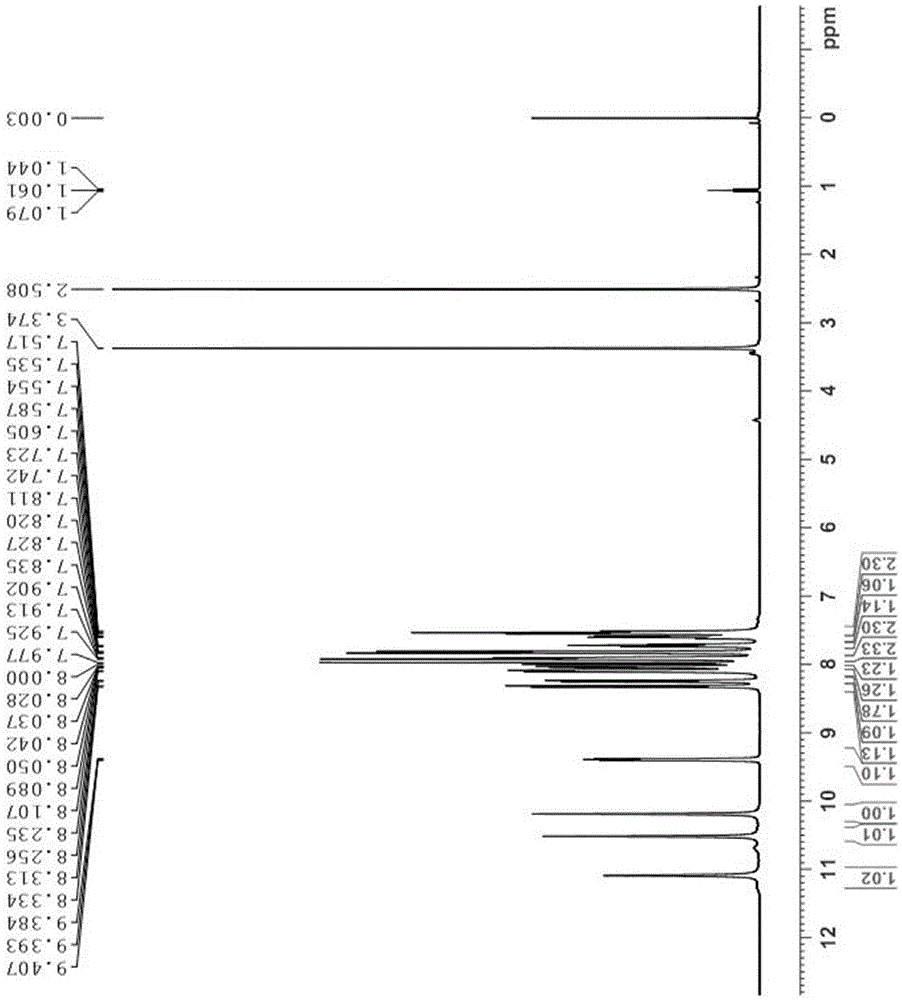
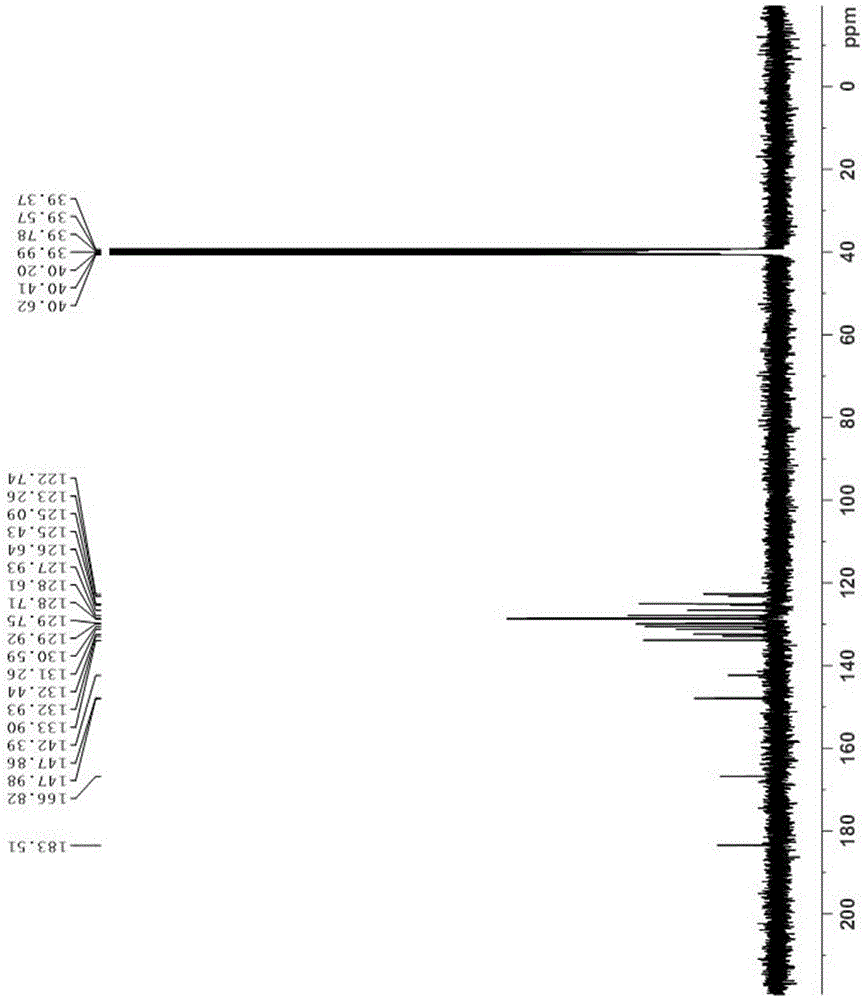
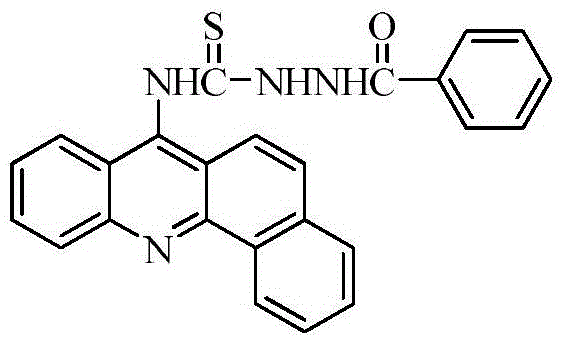
[0026] A benzo(c)acridine derivative, which has the structural formula:
[0027]
[0028] The synthetic route of the benzo(c)acridine derivative is as follows:
[0029]
[0030] The preparation of 7-benzo (c) acridine benzamide thiourea ④, the specific steps are as follows:
[0031] The following raw materials are calculated in parts by weight:
[0032] 1) In a three-necked flask, add 12 parts of o-bromobenzoic acid, 8 parts of naphthylamine, 13 parts of potassium carbonate and 0.9 parts of copper powder, add 65 parts of n-pentanol as a solvent, react at 100°C for 1 hour, and remove under reduced pressure After n-pentanol, add 400 parts of water and react at 60℃ for 20 minutes. After filtering, wash the filter cake with water, collect the washed water and combine the filtrate, acidify with concentrated hydrochloric acid to pH 2, and precipitate a large amount of purple black precipitate , Suction filtration, the obtained black solid is recrystallized with acetone, a...
Example Embodiment
[0039] Example 2
[0040] A benzo(c)acridine derivative, which has the structural formula:
[0041]
[0042] The synthetic route of the benzo(c)acridine derivative is as follows:
[0043]
[0044] The preparation of 7-benzo (c) acridine benzamide thiourea ④, the specific steps are as follows:
[0045] The following raw materials are calculated in parts by weight:
[0046] 1) In a three-necked flask, add 20 parts of o-bromobenzoic acid, 12 parts of naphthylamine, 10 parts of potassium carbonate and 1.0 part of copper powder, add 150 parts of n-pentanol as a solvent, react at 105°C for 1.5 hours, and remove under reduced pressure After n-amyl alcohol, add 1000 parts of water and react at 100℃ for 10 minutes. After filtering, wash the filter cake with water, collect the washed water and combine the filtrate, acidify with concentrated hydrochloric acid to pH 1.5, and precipitate a large amount of purple black precipitate , Suction filtration, the obtained black solid is recrystallized wit...
Example Embodiment
[0051] Example 3
[0052] A benzo(c)acridine derivative, which has the structural formula:
[0053]
[0054] The synthetic route of the benzo(c)acridine derivative is as follows:
[0055]
[0056] The preparation of 7-benzo (c) acridine benzamide thiourea ④, the specific steps are as follows:
[0057] The following raw materials are calculated in parts by weight:
[0058] 1) In a three-neck flask, add 40 parts of o-bromobenzoic acid, 25 parts of naphthylamine, 31 parts of potassium carbonate and 2.5 parts of copper powder, add 100 parts of n-pentanol as a solvent, react at 180°C for 1 hour, and remove under reduced pressure After n-pentanol, add 1100 parts of water and react at 50℃ for 15 minutes. After filtration, wash the filter cake with water, collect the washed water and combine the filtrate, acidify with concentrated hydrochloric acid to pH 2.5, and precipitate a large amount of purple black precipitate , Suction filtration, the obtained black solid is recrystallized with aceton...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com