Novel camptothecin derivative and application thereof to preparation of antitumor drug
An anti-tumor drug, camptothecin technology, applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of poor water solubility, lack of targeting, poor stability, etc., to achieve reduced anti-tumor activity and strong anti-tumor Active, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
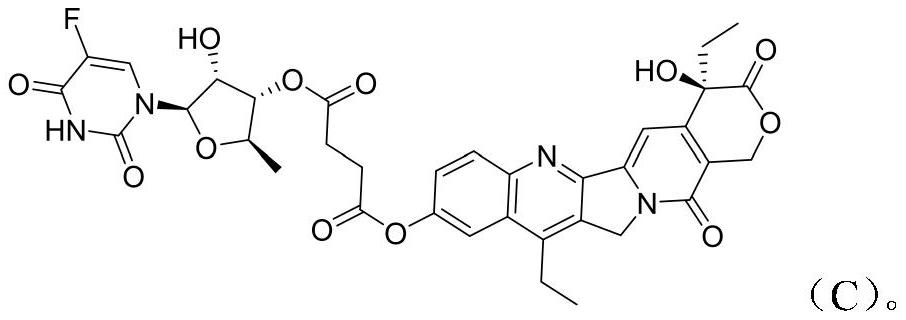
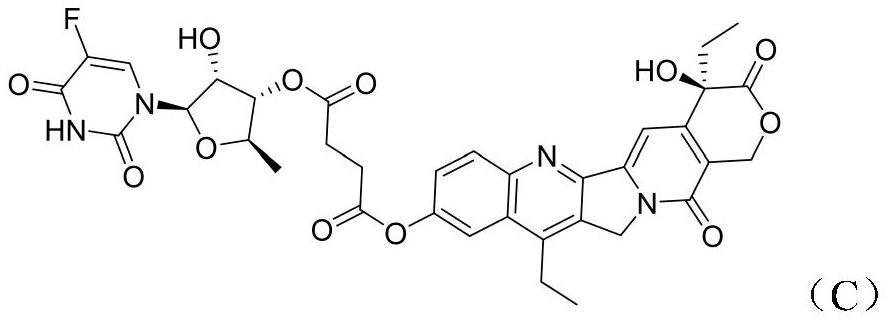
[0020] The synthesis of embodiment 1 novel camptothecin derivative C
[0021] Prepare as follows:
[0022]
[0023] 1) Preparation of compound 7: Add compound 4 (5.42g, 22mmol), acetonitrile (108.4mL), succinic anhydride (2.0g, 20mmol) and 4-dimethylaminopyridine (3.7g, 30mmol) into a 1L flask, The reaction mixture was stirred overnight at room temperature. Concentrate first, then add ethyl acetate to dilute, wash with water, then dry the organic phase with anhydrous sodium sulfate, concentrate to dryness, and the obtained residue is separated by preparative HPLC to obtain compound 7 (2.1 g, 30.3% yield). LC-MS m / z: 347[M+H]+; RT=4.901min.
[0024] 2) Preparation of Compound C: Compound 1 (2.83 g, 7.22 mmol), 1-(3-di Methylaminopropyl)-3-ethylcarbodiimide (2.8 g, 14.4 mmol) and 4-dimethylaminopyridine (88 mg, 0.722 mmol). The reaction mixture was stirred overnight at room temperature. Concentrate first, then add ethyl acetate to dilute, wash with water, then dry the or...
Embodiment 2
[0025] Example 2 Inhibitory experiment of camptothecin derivative C on the proliferation of colorectal cancer LS180, HCT116, HT-29 and CT-26
[0026] The present invention adopts MTT method to study the proliferation inhibitory effect of camptothecin derivative C on colorectal cancer LS180, HCT116, HT-29 and CT-26.
[0027] LS180, HCT116, HT-29 and CT-26 cells were planted on 96-well plates at a density of 3×10 3 / well (100 μL), at 37°C, 5% CO 2 Cells were cultured overnight in an incubator. After 24 hours, the cells were treated with different concentrations of the tested compounds. For LS180 and CT-26 cells, camptothecin derivatives C, SN-38 and CPT-11 were prepared by diluting 10 μM stock solution with RPMI-1640 complete medium. The RPMI-1640 complete medium solution containing 0.1% DMSO was used as the control. For HCT116 and HT-29 cells, the compound was formulated using McCoys 5A complete medium diluted 10 μM stock solution, and McCoys 5A complete medium solution cont...
Embodiment 3
[0034] Embodiment 3 toxicity test
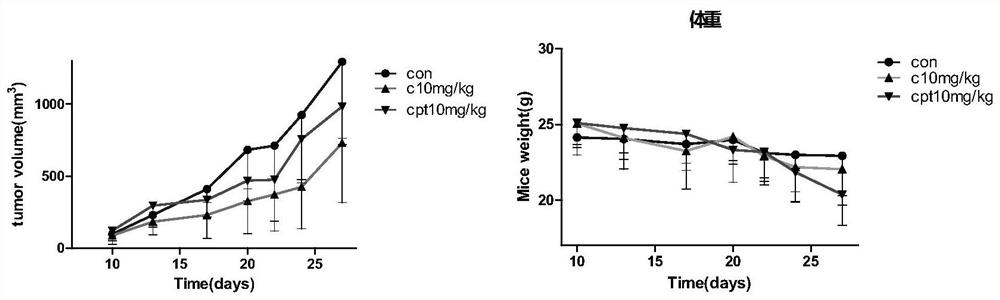
[0035] Human colorectal adenocarcinoma cell line LS180 was used to construct a xenograft tumor model to investigate the antitumor activity of camptothecin derivative C in vivo, and CPT-11 was used as a positive control.
[0036] Nude mice (8 weeks old, BALB / c, male) were used to establish xenograft tumors. LS180 cells (5x10 61) inoculated into the subcutaneous tissue of the right armpit, and injected 100 μL (PBS: Matrigel 1:1). When the tumor volume reaches 100-200mm 3 When left and right, mice were randomly divided into 3 groups, control group, camptothecin derivative C (10 mg / kg), CPT-11 (10 mg / kg), 7 mice in each group. Tumor size and mouse body weight were measured every 3 days. Animals were sacrificed 27 days after tumor cell implantation.
[0037] Test results such as figure 1 As shown: the inhibitory rate of camptothecin derivative C to tumor is greater than that of CPT-11, and the body weight of the animals in the camptothecin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com