Fifteen-nuclear silver cluster taking tri((diphenylphosphine)methene)amine as ligand as well as synthetic method and antitumor application thereof
A synthesis method and cluster technology, applied in the field of medicine, can solve problems affecting ribosomal subunit protein expression, cell apoptosis, damage, etc., and achieve good medicinal value and strong anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Clusters [Ag 15 (N-triphos) 4 (Cl 4 )](NO 3 ) 3 Synthesis
[0026] 1) Dissolve 60mg (0.35mmol) silver nitrate in 0.5mL distilled water, add 2.5mL methanol after complete dissolution, add 30mg (0.05mmol) NP 3 Place on a magnetic stirrer and continue stirring for 30 min. After complete reaction, add 6 mL (0.53 mmol) of newly configured sodium borohydride solution and react at room temperature for 20 h;
[0027] 2) After the reaction, centrifuge in a high-speed centrifuge at 10,000 r / min for 2 minutes, discard the supernatant reaction liquid, dissolve and extract with 7 mL of chloroform, and obtain black crystals after one week of ether vapor phase diffusion, which is the product.
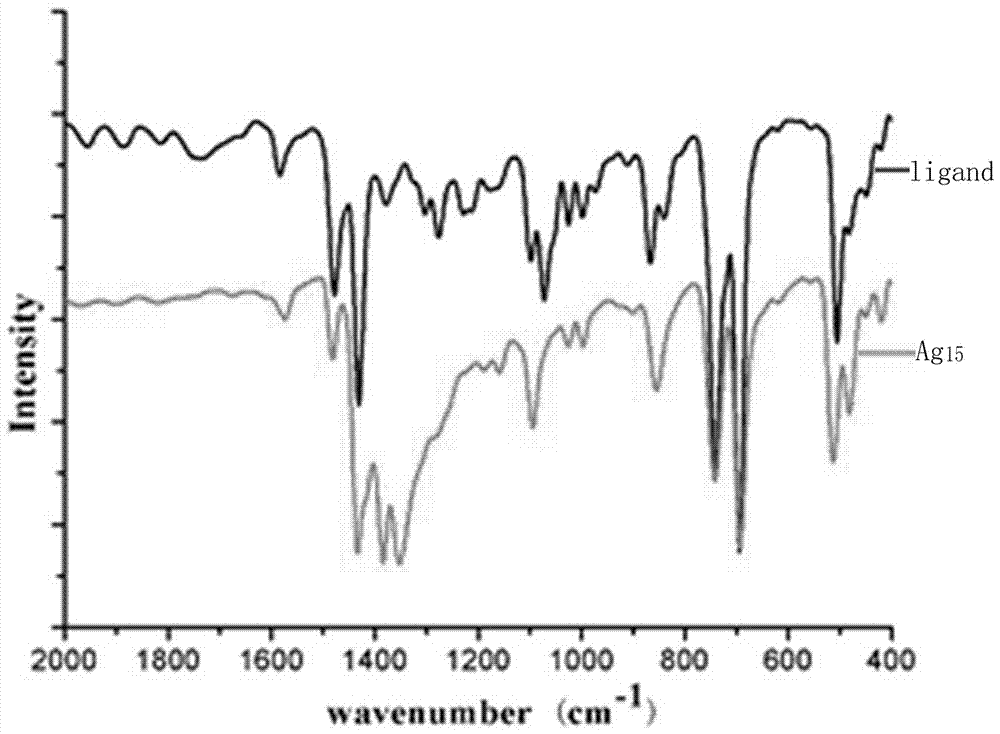
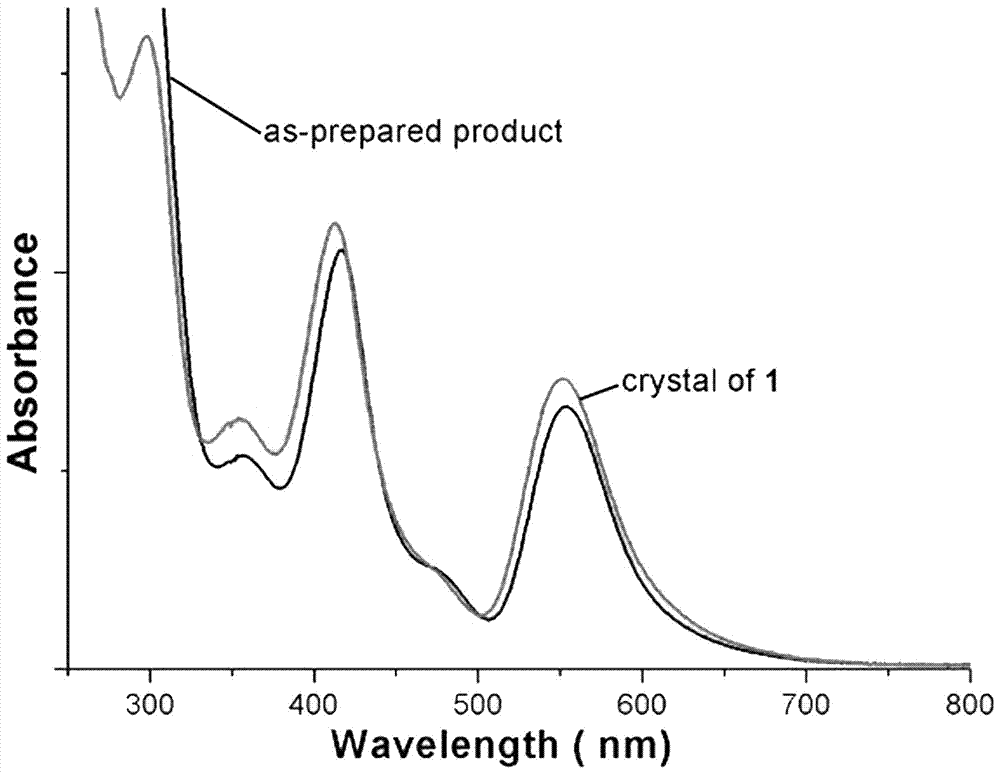
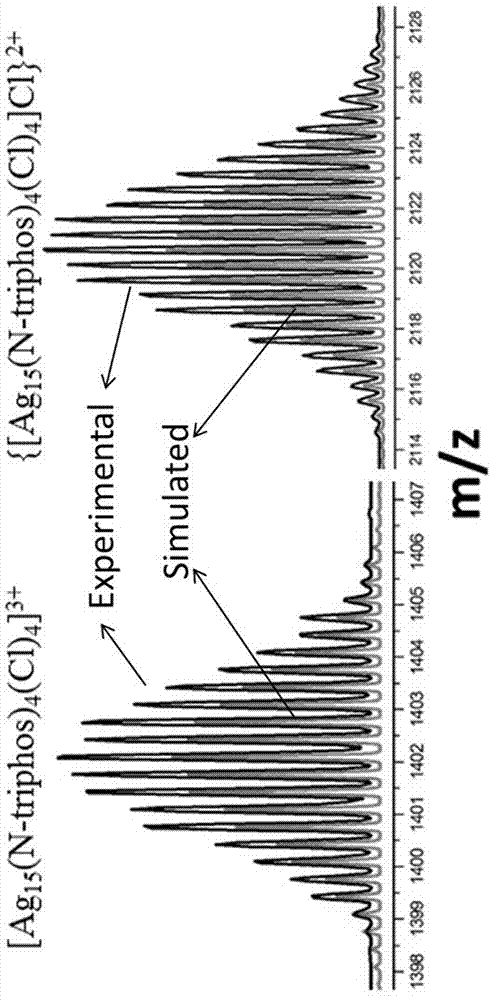
[0028] The obtained black crystals were analyzed by electrospray mass spectrometry and single crystal diffraction. Specific electrospray mass spectrometry data are as follows:
[0029] (1) ESI-MS m / z :1402 [Ag 15 (N-triphos) 4 (Cl) 4 ] 3+ 、2220 {[Ag 15 (N-triphos) 4 ...
Embodiment 2
[0033] 1) Dissolve 60mg (0.35mmol) of silver nitrate in 3mL of ethanol, add 30mg (0.05mmol) of NP3 after complete dissolution, place on a magnetic stirrer and continue stirring for 30min, and add 6mL (0.53mmol) of newly prepared boron after complete reaction Sodium hydride solution, the solution turned from colorless and transparent to black rapidly, and stirred and reacted at room temperature for 20 hours;
[0034] 2) After the reaction, centrifuge in a high-speed centrifuge at 10,000r / min for 2min, discard the supernatant reaction liquid, dissolve and extract with 1mL of anhydrous methanol and 6mL of chloroform, and obtain black crystals after five days of liquid phase diffusion in ether, which is the product. The obtained black crystals were analyzed by electrospray mass spectrometry and single crystal diffraction, and determined to be the target cluster compound [Ag 15 (N-triphos) 4 (Cl 4 )](NO 3 ) 3 .
Embodiment 3
[0036] The difference from Example 2 is that after the reaction, the solvent was spin-dried with a rotary evaporator, and then 7 mL of chloroform was added for dissolution and extraction, and the product was obtained as black crystals by diethyl ether diffusion.
[0037] The obtained black crystals were analyzed by electrospray mass spectrometry and single crystal diffraction, and determined to be the target cluster compound [Ag 15 (N-triphos) 4 (Cl 4 )](NO 3 ) 3 .
[0038] In order to fully illustrate the use of the Ag15 cluster compound with tris((diphenylphosphino)methyl)amine as ligand in pharmaceuticals, an anti-tumor activity experiment was carried out.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com