Nickel nitrate (ii) chelate with 1-pyridine-6-methoxyl-β-carboline as ligand and its synthesis method and application
A synthesis method and technology of nickel nitrate hexahydrate, applied in the field of medicine, can solve the problems of limited preparation cost, complicated extraction, no synthesis method and application, etc., and achieve the effects of good medicinal value and strong anti-tumor activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
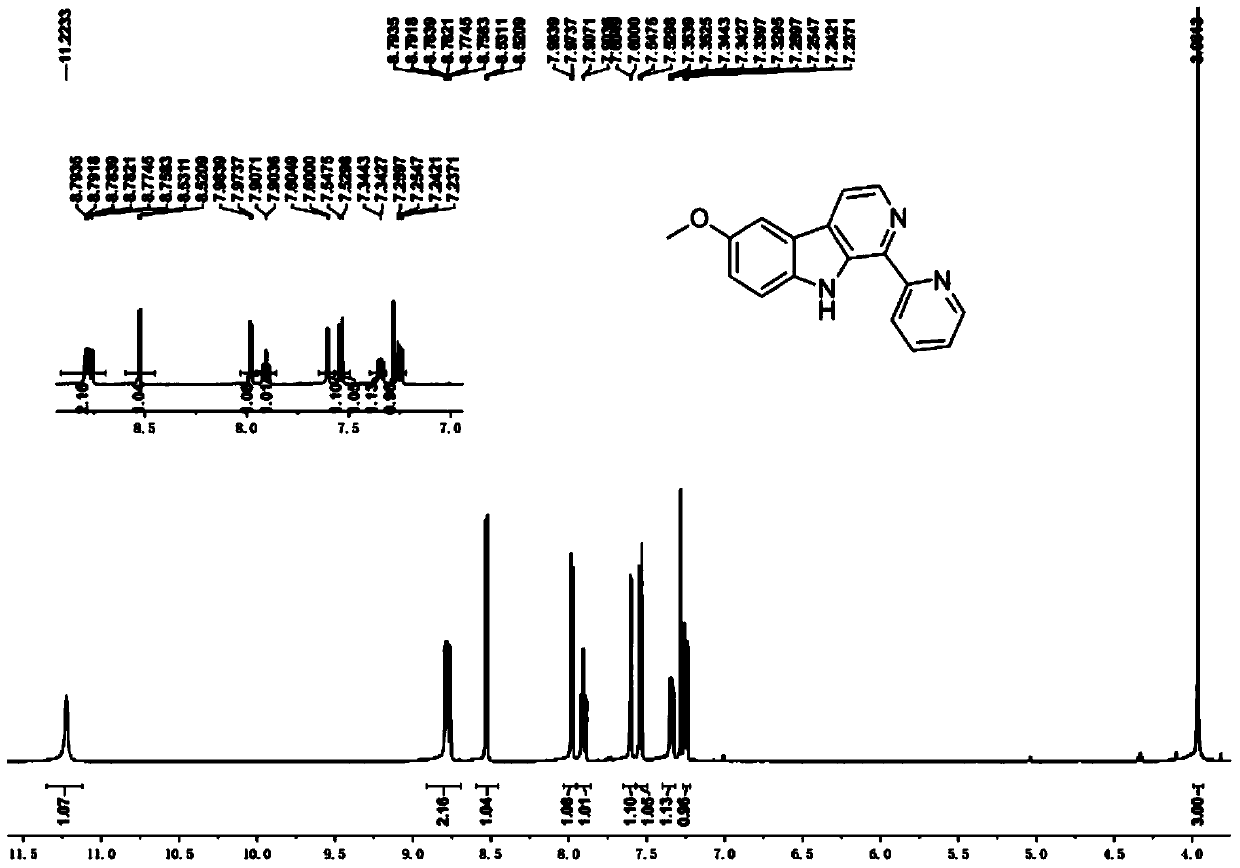
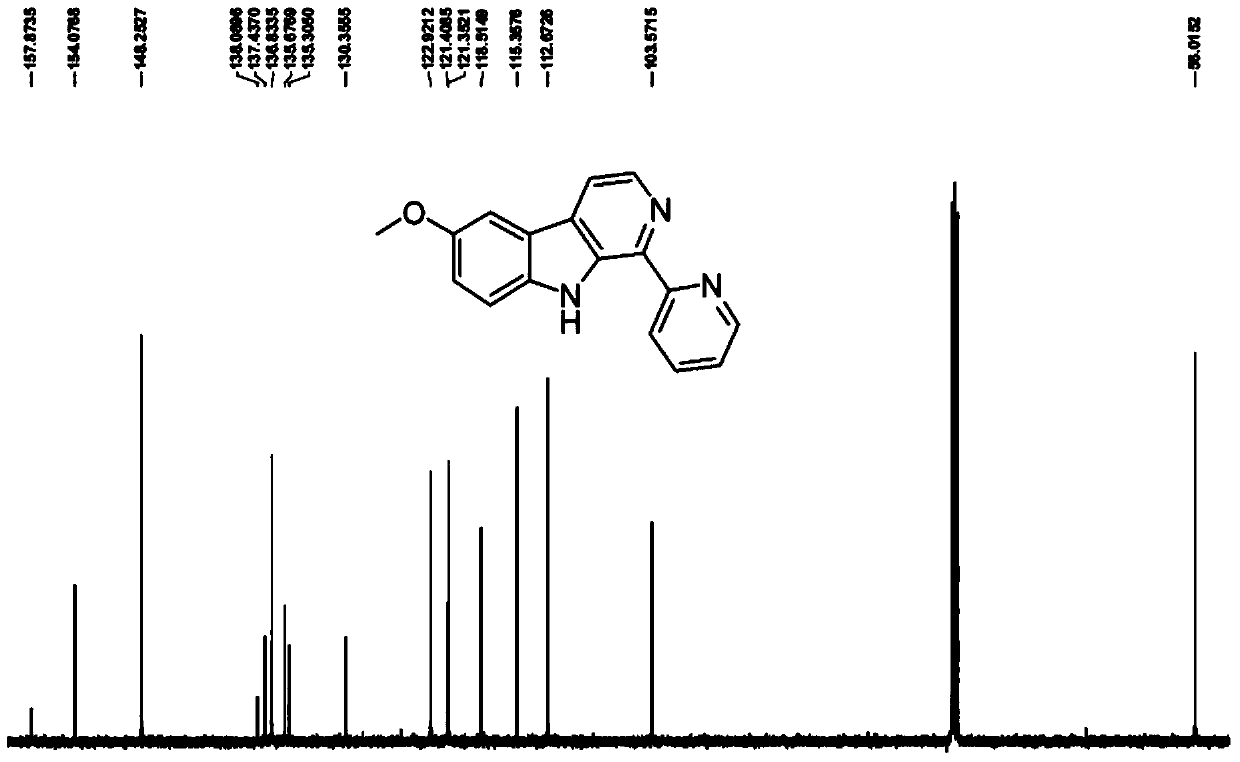
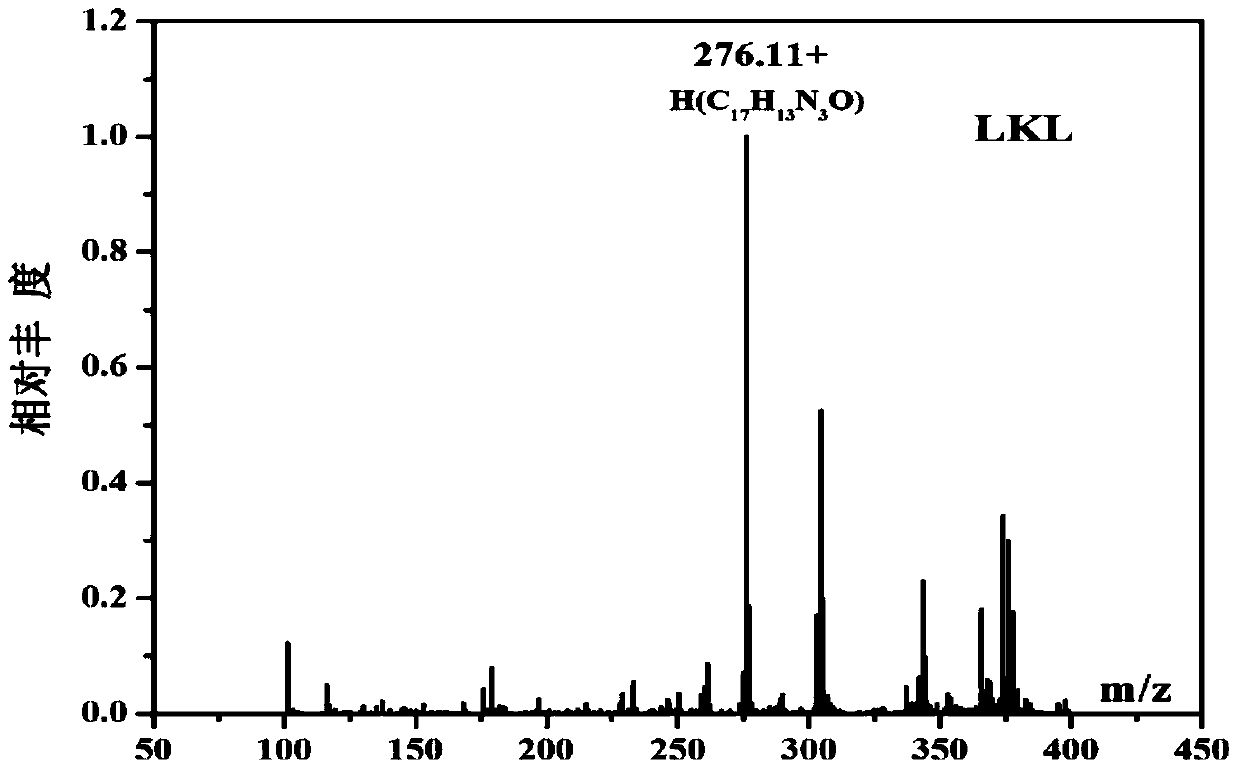
[0043] Embodiment 1: The compound shown in formula (II) is the synthesis of 1-pyridine-6-methoxy-β-carboline (LKL)
[0044] 1) Dissolve 50 mg of 5-methoxytryptamine in 20 mL of tetrahydrofuran, stir and add 25 μL of pyridine-2-carbaldehyde dropwise, stir in ice bath for 20 minutes, then add 110 μL of trifluoroacetic acid, react for about 1 hour, remove the ice bath, Continue the reaction at room temperature for about 1 hour; after the reaction, the obtained reactant is adjusted to neutrality with a sufficient amount of ammonia water, and the organic phase is separated with ethyl acetate, and spin-dried under reduced pressure to obtain a yellow oily liquid, which is directly used in the next step without purification. ;
[0045] 2) Add yellow oily liquid to 50mL xylene, then add 100mg 10% Pd / C, heat up to 140°C, reflux reaction, overnight; after the reaction, filter the reactant, filter cake with methanol, chloroform, acetic acid Ethyl ester was washed 10 times, and the filtra...
Embodiment 2
[0054] Example 2: Synthesis of Ligand LKL
[0055] 1) Take 50 mg of 5-methoxytryptamine, dissolve it in 30 mL of dichloromethane, stir and add 30 μL of pyridine-2-carbaldehyde dropwise, stir evenly for 10 minutes, add 180 μL of acetic acid, and react at room temperature for about 2 hours; The obtained reactant was adjusted to neutrality with a sufficient amount of ammonia water, and the organic phase was separated with chloroform, and spin-dried under reduced pressure to obtain a yellow oily liquid, which was directly used in the next step without purification;
[0056] 2) Add the yellow oily liquid to 40mL of anisole, then add 50mg of 10% Pd / C, heat up to 160°C, and reflux for 20h; Wash 5 times, collect filtrate, filtrate is spin-dried under reduced pressure, obtains crude product, upper fast liquid chromatography afterwards purifies (V 乙酸乙酯 :V 石油醚 =3:9), yellow crystals were obtained (yield about 55%).
[0057] The obtained yellow crystals were analyzed by H NMR, C NMR, e...
Embodiment 3
[0058] Example 3: Synthesis of Ligand LKL
[0059] 1) Dissolve 50 mg of 5-methoxytryptamine in 30 mL of tetrahydrofuran, stir and add 20 μL of pyridine-2-carbaldehyde dropwise, stir in an ice bath for 15 minutes, then add 60 μL of sulfuric acid, react for about 1 hour, remove the ice bath, and continue at room temperature The reaction was about 0.5h; after the reaction, the obtained reactant was adjusted to be alkaline with a sufficient amount of ammonia water, and the organic phase was separated with ethyl acetate, and spin-dried under reduced pressure to obtain a yellow oily liquid, which was directly used in the next step without purification;
[0060] 2) Add the yellow oily liquid to 40mL of anisole, then add 120mg of 5% Pd / C, heat up to 150°C, and reflux for 24h; Ethyl ester was washed 8 times successively, and the filtrate was collected, and the filtrate was spin-dried under reduced pressure to obtain a crude product, which was then purified by flash liquid chromatograph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com