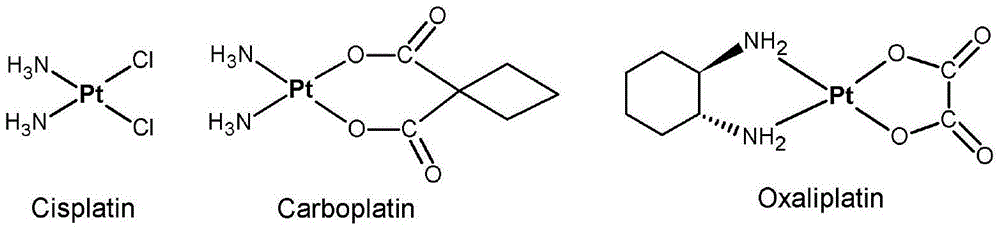
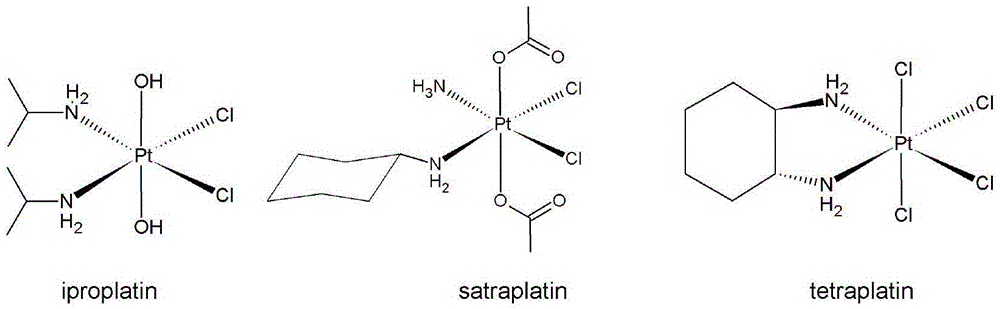
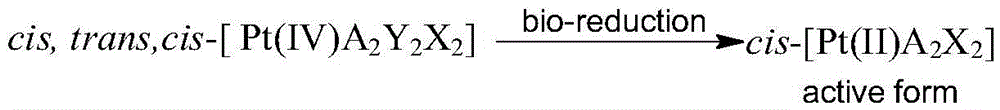Platinum (IV) anticancer compounds with dihydrogen phosphate radical as axial ligand
A dihydrogen phosphate compound technology, applied in the field of biopharmaceuticals, can solve problems such as poor water solubility, poor anticancer activity, and failure to be approved for marketing, and achieve good selectivity, high anticancer activity, and good clinical application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0041] (1) The synthetic method of Pt (IV) anticancer compound PPt1 of the present invention
[0042] Weigh 8.40g (28mmol) cisplatin, add 250ml of water, stir, heat to 60°C, add 30wt% H 2 o 2 420ml, continue to stir and react at 60°C for 4 hours, cool to room temperature, precipitate a light yellow product, filter, wash with ice water, dry, and recrystallize in water to obtain light yellow crystals of cis, trans, cis-[ Pt(IV)(NH 3 ) 2 (OH) 2 Cl 2 ]5.8g, the yield is about 62%.
[0043] Accurately weigh 2.061g (6.17mmol) cis, trans, cis-[Pt(IV)(NH 3 ) 2 (OH) 2 Cl 2 ], add 0.678 grams of 85% phosphoric acid solution (5.88mmol, be equivalent to 95% of stoichiometry), after stirring and mixing, add 50ml of water stirring reaction again at 60 ℃ and after concentrating under reduced pressure to near dryness, add 100ml of Stir to dissolve in water, cool to room temperature, filter out unreacted cis, trans, cis-[Pt(IV)(NH 3 ) 2 (OH) 2 Cl 2 ], the filtrate was freeze-drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com