Compositions and methods of treating liver cancer
A technology of liver cancer and radiotherapy, applied in the field of compositions and methods for treating liver cancer, capable of solving problems such as inapplicability of surgical resection, deterioration of cases, and complicated treatment of liver cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
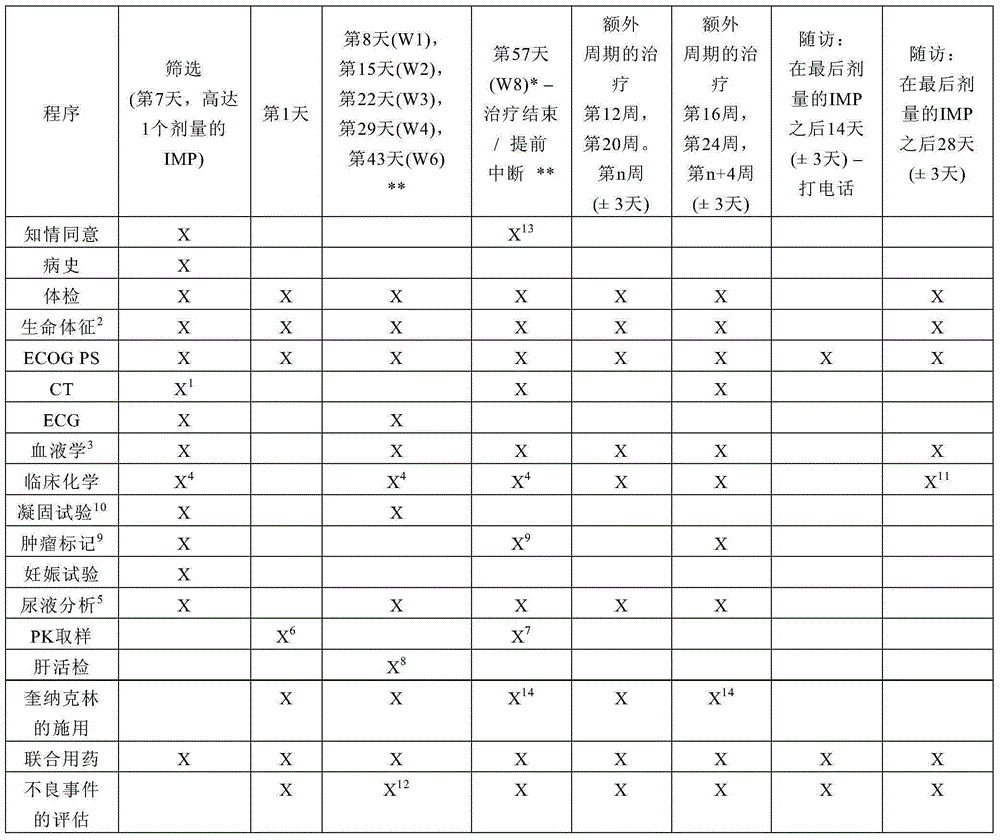
[0093] Example 1: Study Plan (Description of General Study Design and Plan)
[0094] After signing the informed consent, the patients underwent a screening clinical examination. Compliance with inclusion / exclusion was confirmed prior to study therapy.
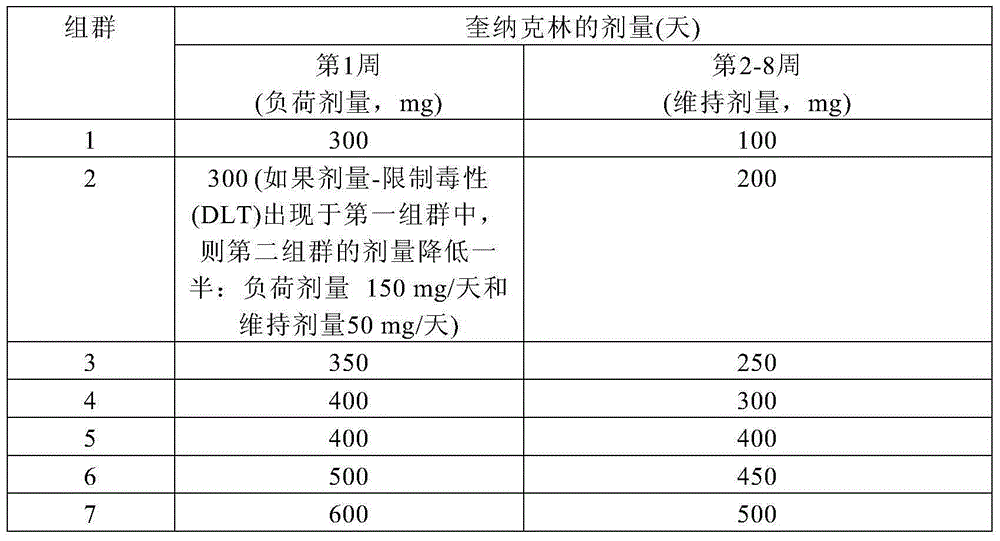
[0095] Study subjects were consecutively enrolled into 7 dosing cohorts. The dose was increased with each subsequent cohort. First, patients take a loading dose. During the following weeks of the study (starting at week 2), the dose was reduced and patients were on a maintenance dose until study completion (Table 1). Patients in Cohort 5 took the same dose throughout the 8-week treatment period.
[0096] Table 1. Dosing regimen for quinacrine
[0097]
[0098] At least 3 patients were enrolled in each cohort. If no patients experienced dose-limiting toxicities (DLTs) after 4 weeks of treatment, 3 additional patients were immediately enrolled in the next cohort. If any patient prematurely discontinued study therapy fro...
Embodiment 2
[0145] Example 2: Statistical Methods and Sample Size Determination Specified by the Protocol
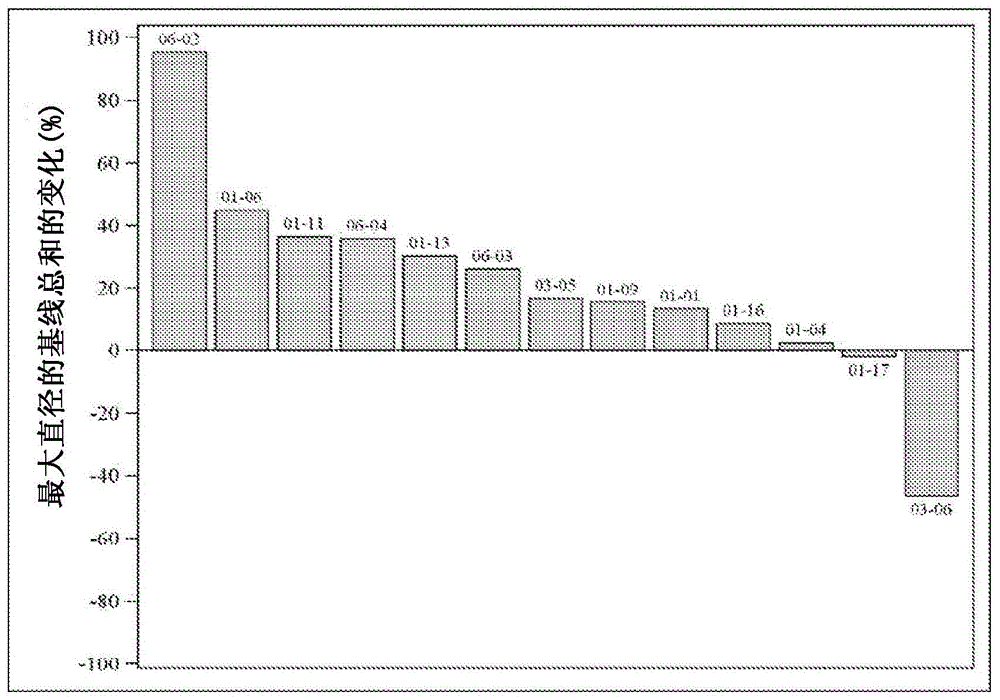
[0146] Statistics and Analytical Protocols With regard to illustrative study objectives such as determination of dose-limiting toxicities, assessment of safety, tolerability and pharmacokinetics, the primary means of analysis were descriptive statistics and graphical representations. Summarize and describe numerical data with the following parameters: number of observations, number of missed observations, minimum, maximum, arithmetic mean, standard deviation, median, mode, 25th percentile, and 75th percentile. For the assessment of qualitative data, the number and percentage age of cases are presented. Measurements / exams performed prior to the first dose of study product (at the Day 1 visit) were considered baseline data. The change from baseline was calculated as the difference between the measurement / examination at Visit Х and the measurement / examination at the Day 1 visit. The ...
Embodiment 3
[0149] Example 3: Treatment
[0150] Oral quinacrine (capsule 50mg). The dosing regimen depends on the dose prescribed to the patient according to the study cohort.
[0151] Group 1
[0152] Days 1-7 Loading dose – 100 mg (2 capsules) three times a day.
[0153] Days 8-56 Maintenance dose – 100 mg (2 capsules) once daily in the morning.
[0154] Group 2
[0155] Days 1-7 Loading dose – 100 mg (2 capsules) three times a day.
[0156] Days 8-56 Maintenance dose – 100mg (2 capsules) in the morning and 100mg (2 capsules) within 4 hours.
[0157] If DLT occurs in the first cohort, reduce the dose in the second cohort to 1 / 2: loading dose 150 mg per day and maintenance dose 50 mg per day.
[0158] Group 3
[0159] Days 1-7 Loading dose – 150 mg (3 capsules) in the morning, 100 mg (2 capsules) in the afternoon, and 100 mg (2 capsules) in the evening.
[0160] Days 8-56 Maintenance dose – 150mg (3 capsules) in the morning and 100mg (2 capsules) within 4 hours.
[0161] Group ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com