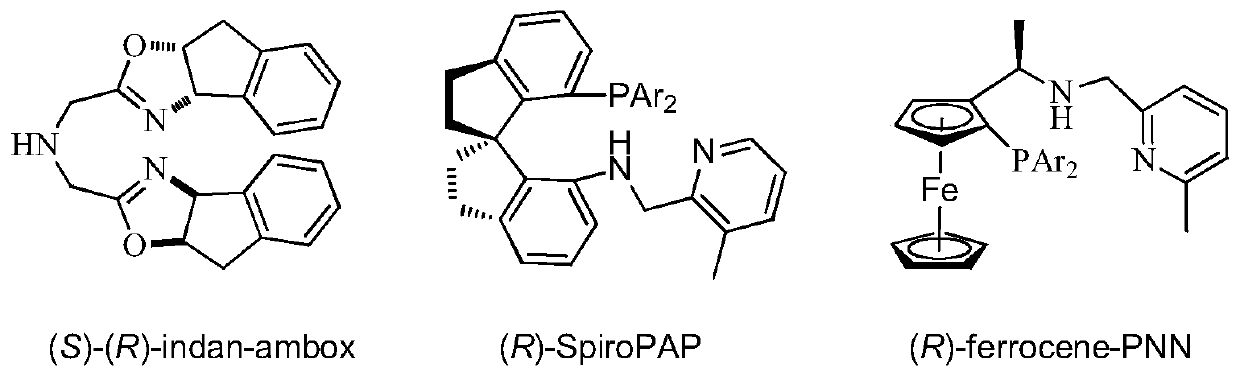
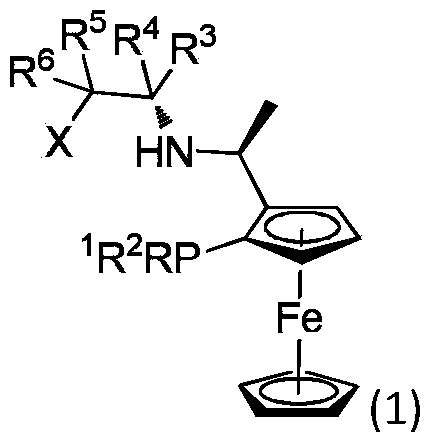
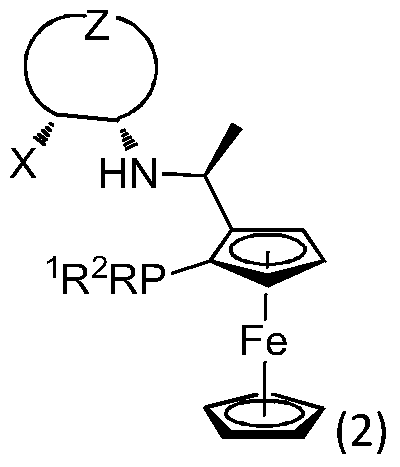
Application of a Chiral Tridentate Phosphine Nitrogen Oxygen Ligand and Its Related Ligands in Asymmetric Catalytic Reactions
An asymmetric reaction technology, applied in catalytic reactions, organic compound/hydride/coordination complex catalysts, preparation of organic compounds, etc., can solve the problems of complex and expensive ligand synthesis, and achieve easy purification, Strong nucleophilicity, suitable for large-scale synthesis and preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis of ligand L4:
[0058]
[0059] (S)-Ugi-amine 1 (2.57g, 10mmol) dissolved in 20mL of anhydrous ether, N 2 Protected, tert-butyllithium (6.9 mL, 1.6M n-pentane solution) was added dropwise under the condition of -78°C, after the addition, stirred at -78°C for half an hour, and then brought to room temperature for lithiation for 1 hour. Diphenylphosphine chloride (4.41g, 20mmol) was slowly dropped at 0°C. After the addition is complete, reflux and react for 2h. Under ice-water bath, the reaction was quenched with saturated sodium bicarbonate solution, the aqueous phase was extracted with ether, the organic phases were combined, washed with water, dried over anhydrous sodium sulfate, concentrated, column chromatography, ethanol recrystallized to obtain 2.92g orange solid, yield 66 %.
[0060]
[0061] N 2 Under the atmosphere, 2 (2.2g, 5mmol) and 3.2mL of anhydrous acetic anhydride were added to the reaction tube, and the reaction was refluxed at 100°C for 2h. Afte...
Embodiment 2
[0066] Synthesis of ligand L8:
[0067]
[0068] (S)-Ugi-amine 1 (2.57g, 10mmol) dissolved in 20mL of anhydrous ether, N 2 Protected, tert-butyllithium (6.9 mL, 1.6M n-pentane solution) was added dropwise under the condition of -78°C, after the addition, stirred at -78°C for half an hour, and then brought to room temperature for lithiation for 1 hour. Diphenylphosphine chloride (4.41g, 20mmol) was slowly dropped at 0°C. After the addition is complete, reflux and react for 2h. Under ice-water bath, the reaction was quenched with saturated sodium bicarbonate solution, the aqueous phase was extracted with ether, the organic phases were combined, washed with water, dried over anhydrous sodium sulfate, concentrated, column chromatography, ethanol recrystallized to obtain 2.92g orange solid, yield 66 %.
[0069]
[0070] N 2 Under the atmosphere, 2 (2.2g, 5mmol) and 3.2mL of anhydrous acetic anhydride were added to the reaction tube, and the reaction was refluxed at 100°C for 2h. Afte...
Embodiment 3
[0077] Synthesis of ligand L17:
[0078]
[0079] In a 20mL reaction tube, add 0.4563g (1mmol) acetate 3 and 0.7460g (5mmol) (1S,2R)-(-)1-amino-2-indanol, replacing N 2 Then, 10 mL of anhydrous methanol was added, the reaction was carried out at reflux overnight, rotary evaporation was concentrated, and 0.428 g of a pale yellow solid was obtained by column chromatography with a yield of 78%.
[0080] 1 H NMR(400MHz, CDCl 3 )δ7.61-7.50(m,2H),7.44-7.34(m,3H),7.25-7.14(m,5H),7.11-7.02(m,2H),6.86-6.75(m,1H),6.35- 6.23(d,J=7.6Hz 1H),4.58-4.53(m,1H),4.41-4.31(m,2H),4.24-4.18(m,1H),4.04(d,J=5.2Hz,1H), 3.99(s,5H),3.90-3.85(m,1H),2.92-2.82(dd,J=5.2Hz,16.4Hz,1H),2.81-2.73(dd,J=1.6Hz,16.4Hz,1H), 1.58(d,J=6.4Hz,3H); 31 P NMR(162MHz, CDCl 3 )δ-26.11(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com