Adverse drug reaction early warning and analyzing system and method
A technology of adverse reaction and early warning analysis, applied in the medical field, can solve problems such as single function and inconsistency of monitoring results on-site experience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1. An early warning analysis system for adverse drug reactions. Combine below Figure 1 to Figure 2 The system provided in this embodiment will be described in detail.
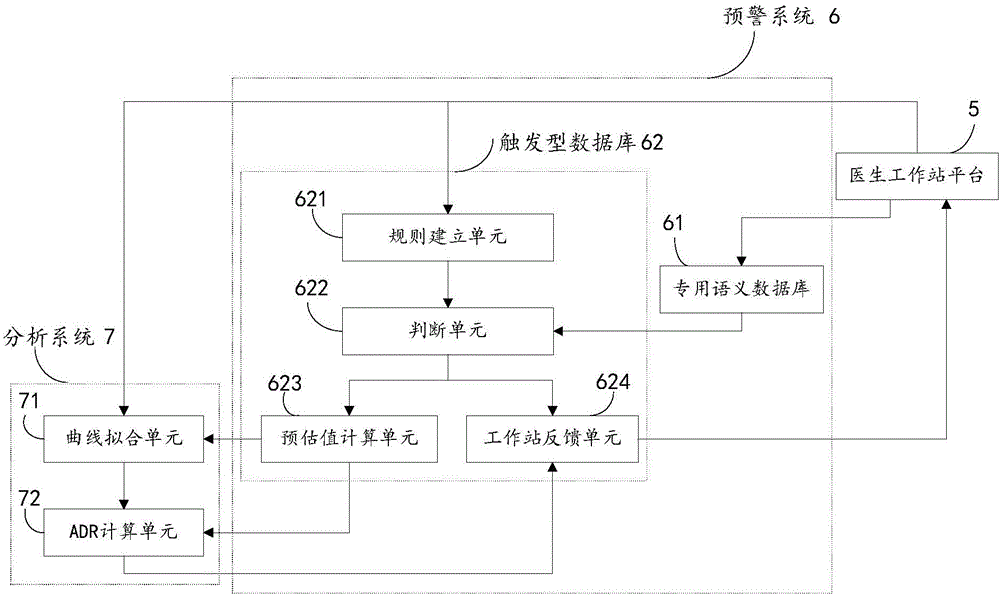
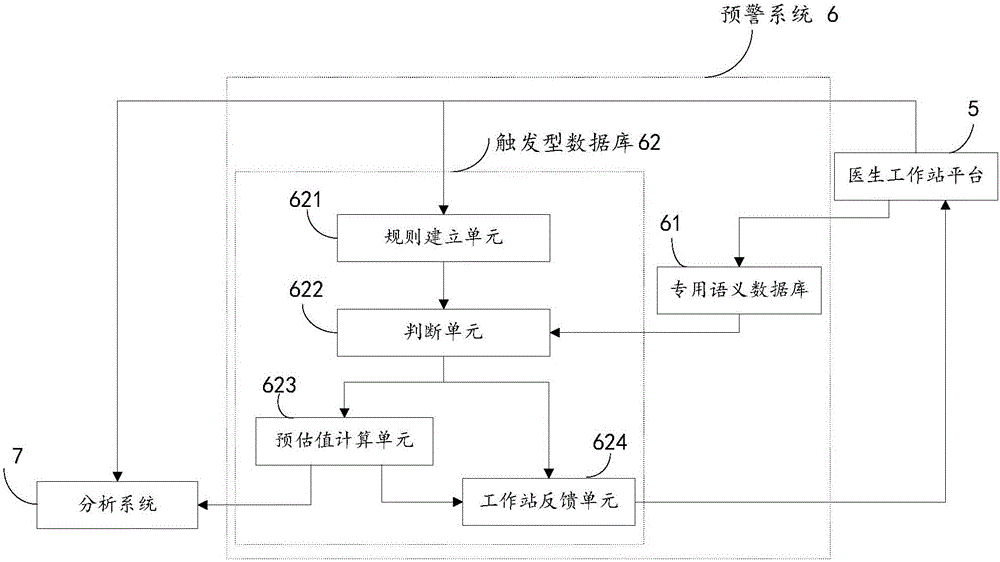
[0026] see figure 1 The present embodiment provides an early warning analysis system for adverse drug reactions, the system includes a doctor workstation platform 5, an early warning system 6, and an analysis system 7, and the early warning system 6 specifically includes a dedicated semantic database 61 and a trigger database 62; The trigger database 62 specifically includes a rule establishment unit 621 , a judgment unit 622 , an estimated value calculation unit 623 and a workstation feedback unit 624 ; the analysis system 7 specifically includes a curve fitting unit 71 and an ADR calculation unit 72 .
[0027] Specifically, the doctor workstation platform 5 is used to send medical record information to the early warning system, and the medical record information includes at least patient ...
Embodiment 2
[0044] Embodiment 2. A method for early warning analysis of adverse drug reactions. Combine below Figure 3 to Figure 5 The method provided in this embodiment will be described in detail.
[0045] see Figure 3 to Figure 4 , S1. The doctor workstation platform sends the medical record information to the early warning system, and the medical record information at least includes the patient's electronic medical record information and medication orders.
[0046] Specifically, the doctor workstation platform sends the medical record information to the dedicated semantic database in the early warning system. The medical record information includes at least the patient's electronic medical record information and medication orders. The patient's electronic medical record information includes the patient's disease condition, combined medication, clinical Information such as test values, age, gender, and allergy history.
[0047] S2. The special semantic database in the early warnin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com