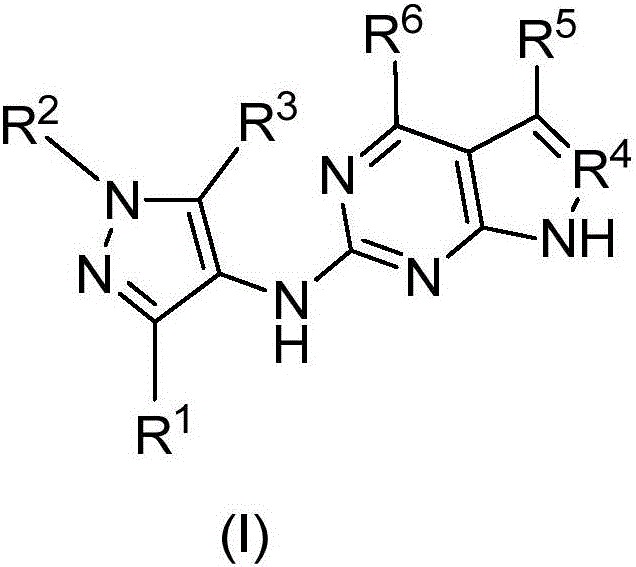
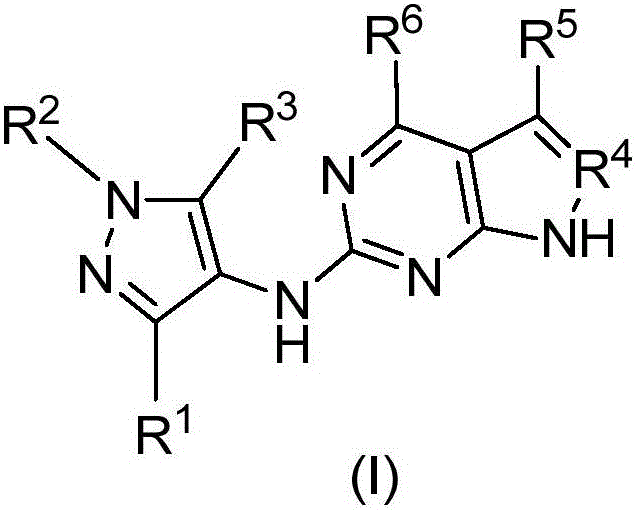
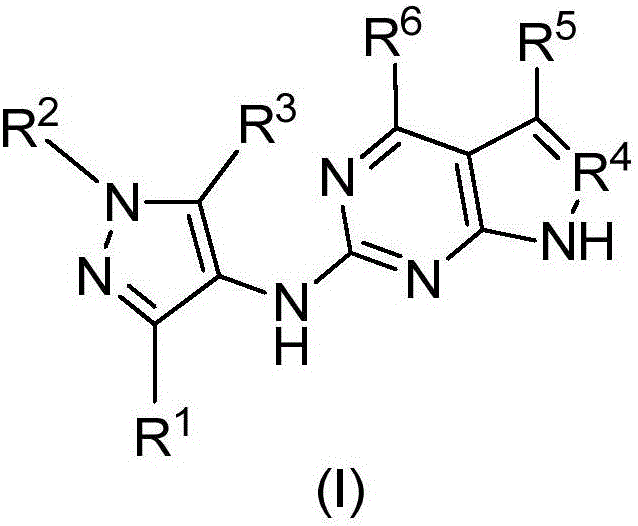
Compounds
A compound and pharmaceutical technology, applied in the field of compounds, can solve problems such as affecting cell degradation of α-synuclein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[2298] N-(1,3-Dimethyl-1H-pyrazol-4-yl)-4-ethoxy-7H-pyrrolo[2,3-d]pyrimidin-2-amine (E1)
[2299]
[2300] N-(1,3-Dimethyl-1H-pyrazol-4-yl)-4-ethoxy-7-toluenesulfonyl-7H-pyrrolo[2,3-d]pyrimidin-2-amine A solution of (which can be prepared according to D3) (100 mg, 0.234 mmol) and sodium hydroxide (0.586 mL, 1.172 mmol) in isopropanol (10 mL) was stirred at 50 °C overnight. The reaction mixture was concentrated in vacuo. The residue was poured into water and extracted with EtOAc (20 mL x 3). The combined organic layers were washed with Na 2 SO 4 Dry, filter and concentrate in vacuo. The crude product was purified by preparative HPLC to afford the title compound E1 (12 mg, 0.042 mmol, 18.12% yield) as a yellow solid.
[2301] LCMS: 273.1[M+H] + . t R = 1.10 min. (LCMS condition 2)
[2302] 1 H NMR (400MHz, chloroform-d): δ8.66-9.05(m, 1H), 7.80(s, 1H), 6.69(d, J=1.5Hz, 1H), 6.41(d, J=1.8Hz, 1H ), 6.24(s, 1H), 4.53(q, J=7.1Hz, 2H), 3.82(s, 3H), 2.27(s, 3H), 1.47(t,...
Embodiment 2 and 3
[2304] 1-(4-((4-ethoxy-7H-pyrrolo[2,3-d]pyrimidin-2-yl)amino)-3-methyl-1H-pyrazol-1-yl)-2- Methylpropan-2-ol (E2)
[2305] 1-(4-((4-ethoxy-7H-pyrrolo[2,3-d]pyrimidin-2-yl)amino)-5-methyl-1H-pyrazol-1-yl)-2- Methylpropan-2-ol (E3)
[2306]
[2307] 2-Chloro-4-ethoxy-7H-pyrrolo[2,3-d]pyrimidine (which can be prepared according to D1) (200 mg, 1.012 mmol), 1-(4-amino-3-methyl-1H -pyrazol-1-yl)-2-methylpropan-2-ol and 1-(4-amino-5-methyl-1H-pyrazol-1-yl)-2-methylpropan-2-ol Mixture (206mg, 1.214mmol), Pd 2 (dba) 3 (46.3mg, 0.051mmol), (9,9-dimethyl-9H-xanthene-4,5-diyl)bis-(diphenylphosphine) (58.6mg, 0.101mmol) and potassium carbonate (420mg, 3.04 mmol) in 2-butanol (2.0 mL) was microwave irradiated at 120° C. for 45 min. The reaction mixture was quenched with water and extracted with EtOAc (20 mL x 3). The combined organic layers were washed with Na 2 SO 4 Dry, filter and concentrate in vacuo. The crude product was purified by silica gel column chromatography (DCM:M...
Embodiment 4
[2313]N-(5-chloro-1-methyl-1H-pyrazol-4-yl)-4-ethoxy-7H-pyrrolo[2,3-d]-pyrimidin-2-amine (E4)
[2314]
[2315] N-(5-chloro-1-methyl-1H-pyrazol-4-yl)-4-ethoxy-7-tosyl-7H-pyrrolo[2,3-d]-pyrimidine-2 - A solution of the amine (which can be prepared according to D4) (50 mg, 0.112 mmol) and sodium hydroxide (1 mL, 2.0 mmol, 2M in water) in methanol (3 mL) was stirred at 50 °C for 2 hours. The mixture was extracted with ethyl acetate. The organic layer was dried and evaporated. The crude product was purified by preparative HPLC to afford the title compound E4 (19 mg, 0.065 mmol, 58.0% yield) as a white solid.
[2316] LCMS: 293[M+H] + . t R = 1.278 minutes. (LCMS condition 2)
[2317] 1 H NMR (400MHz, methanol-d 4 ): δ7.82-8.01 (m, 1H), 6.86 (d, J = 3.5Hz, 1H), 6.32 (d, J = 3.5Hz, 1H), 4.51 (q, J = 7.0Hz, 2H), 3.85 (s, 3H), 1.44 (t, J=7.2Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com