Method for detecting related substances in ketoconazole sample and method for preparing ketoconazole preparation
A related substance, ketoconazole technology, applied in the field of medicine, can solve problems to be improved, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] In this embodiment, 10 microliters of the system suitability solution was taken according to the method and conditions basically the same as the general method, and injected into a high performance liquid chromatograph to detect the system suitability solution. The difference is that the mobile phase ratio used in this embodiment carries out gradient elution according to the following conditions:
[0061]
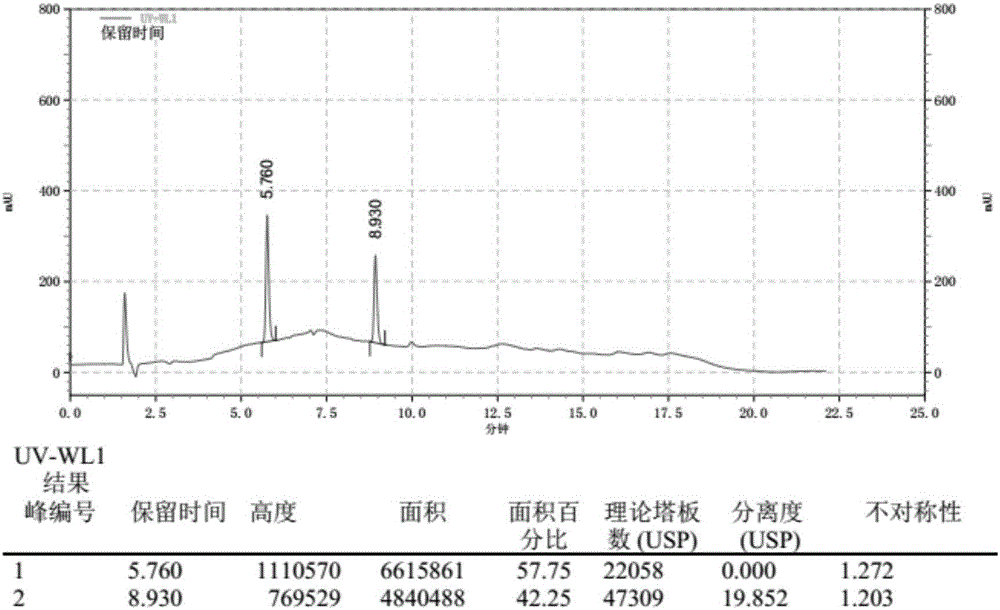
[0062] The chromatogram that this embodiment detects obtains is as figure 1 shown. Depend on figure 1 It can be seen that the retention time of the ketoconazole peak is 5.760 minutes, the retention time of the loperamide hydrochloride peak is 8.930 minutes, and the degree of separation of the two is 19.852, which basically meets the retention time of the ketoconazole peak as 6 minutes, two The detection standard of "Chinese Pharmacopoeia" with a peak resolution greater than 15.
Embodiment 2
[0064] In this embodiment, 10 microliters of the system suitability solution was taken according to the method and conditions basically the same as the general method, and injected into a high performance liquid chromatograph to detect the system suitability solution. The difference is that the mobile phase ratio used in this embodiment carries out gradient elution according to the following conditions:
[0065]
[0066]
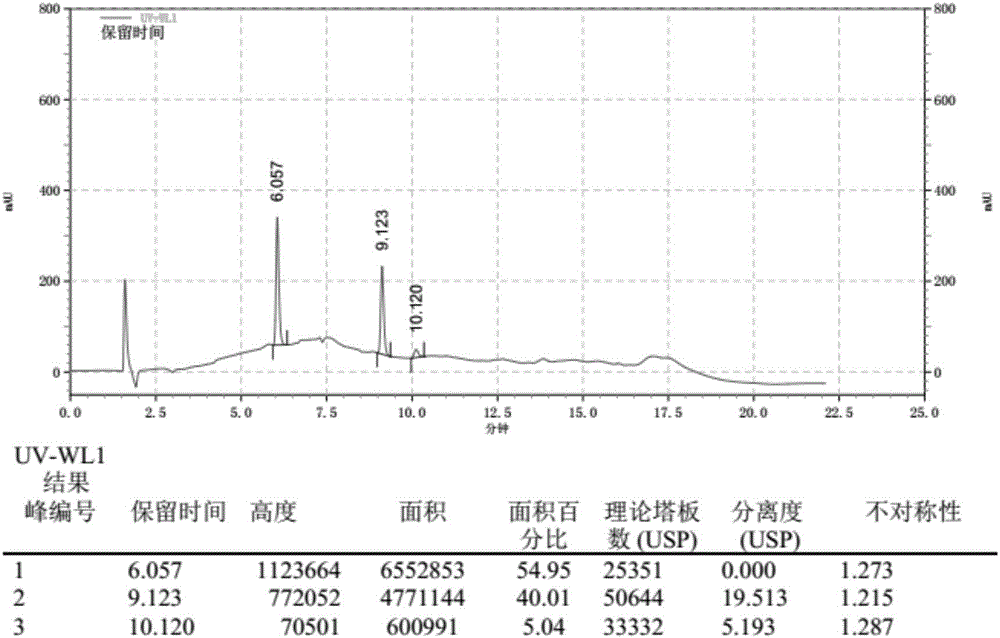
[0067] The chromatogram that this embodiment detects obtains is as figure 2 shown. Depend on figure 2 It can be seen that the retention time of the ketoconazole peak is 6.057 minutes, the retention time of the loperamide hydrochloride peak is 9.123 minutes, and the degree of separation between the two is 19.513, which fully meets the retention time of the ketoconazole peak and is 6 minutes, two The detection standard of "Chinese Pharmacopoeia" with a peak resolution greater than 15.
Embodiment 3
[0069] In this embodiment, 10 microliters of the system suitability solution was taken according to the method and conditions basically the same as the general method, and injected into a high-performance liquid chromatograph to detect the system suitability solution. The difference is that the mobile phase ratio used in this embodiment carries out gradient elution according to the following conditions:
[0070]
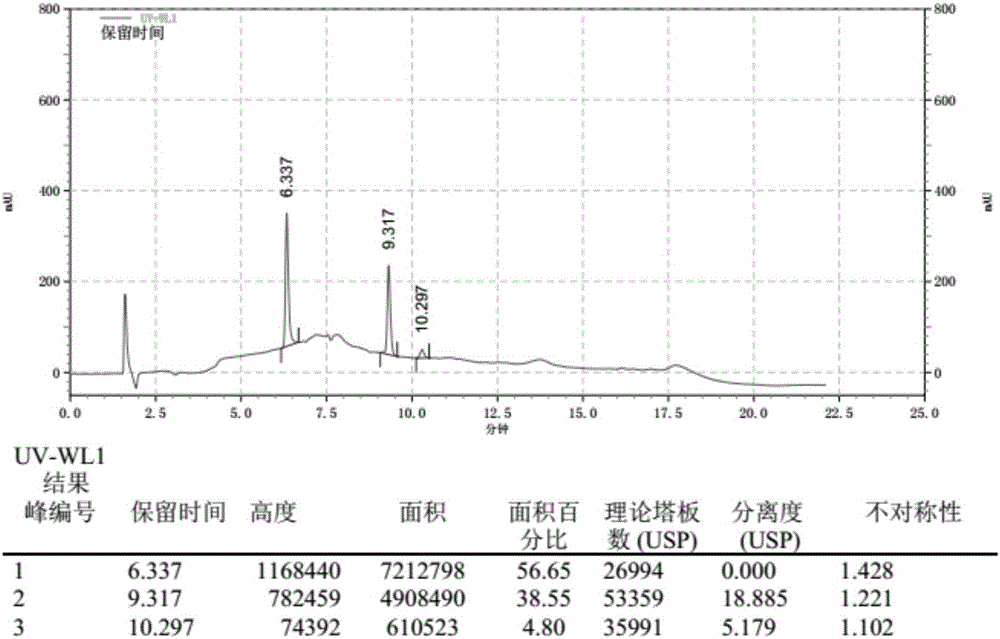
[0071] The chromatogram that this embodiment detects obtains is as image 3 shown. Depend on image 3 It can be seen that the retention time of the ketoconazole peak is 6.337 minutes, the retention time of the loperamide hydrochloride peak is 9.317 minutes, and the degree of separation of the two is 18.885, which basically meets the retention time of the ketoconazole peak as 6 minutes, two The detection standard of "Chinese Pharmacopoeia" with a peak resolution greater than 15.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com