Protein TRIM27 resistant to mycobacterium tuberculosis infection
A mycobacterial, anti-tuberculosis technology, applied in the field of cell biology, can solve problems such as unclear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 TRIM27 protein against mycobacterial infection
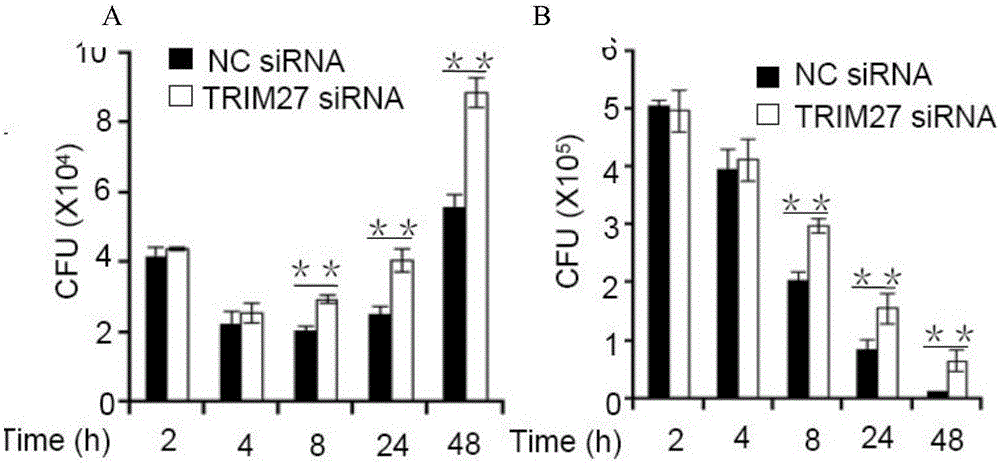
[0016] (A) Experimental method: Infect wild-type and TRIM27-knockout macrophage U937 with Mycobacterium smegmatis (M.smegmatis) and Bacillus Calmette-Guerin (BCG), respectively. CFU changes to prove that TRIM27 plays a role in the process of resisting pathogenic bacteria infection.
[0017] (B) Results: The number of CFU of macrophage U937 cells infected by Mycobacterium smegmatis M.smegmatis was the highest after 2 hours, and the mycobacteria were gradually cleared as the infection time prolonged ( figure 1 B). After BCG was infected with U937, the number of CFU gradually decreased within 8 hours, and the number of CFU began to increase after 24 hours. The reason is that BCG contains many effector proteins that are the same as those of Mycobacterium tuberculosis, which began to interfere with the host's natural immune response and promote BCG. Intrasurvival ( figure 1 A). Comparing the CFU difference betwee...
Embodiment 2
[0018] Example 2 TRIM27 promotes the secretion of inflammatory cytokines
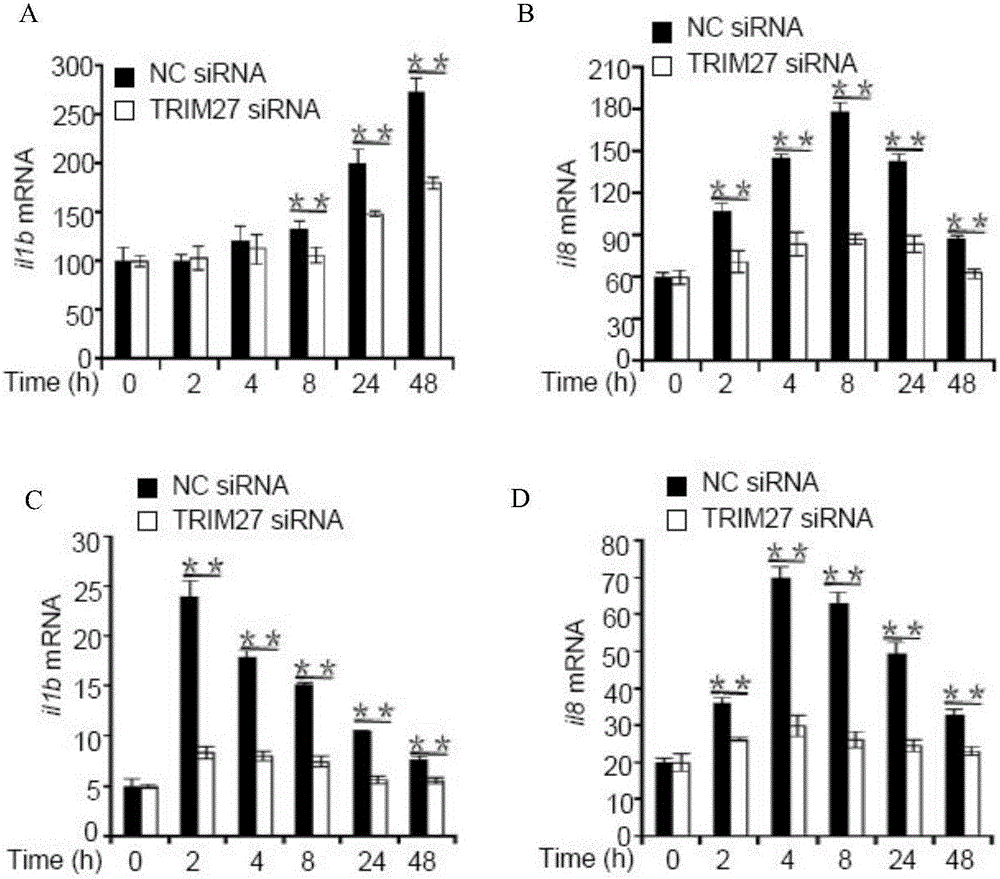
[0019] (A) Experimental method: use M.smegmatis ( figure 2 C,D) and BCG ( figure 2 A, B) Macrophages U937 with wild type and TRIM27 knockout were infected respectively, and the gene transcription levels of cytokines il8 and il1b at different infection time points were detected by fluorescent real-time quantitative PCR.
[0020] (B) Results: Comparing the gene transcription levels of il8 and il1b in wild-type and TRIM27-knockout U937 cells at different infection time points, the results showed that during the infection of M. and il1b gene transcription levels were significantly reduced. Therefore, during mycobacterial infection of macrophages, TRIM27 plays an important role in promoting the secretion of inflammatory cytokines to resist pathogenic infection.
Embodiment 3
[0021] Example 3 TRIM27 Promotes the Apoptotic Process of Pathogenic Bacteria Infected Cells
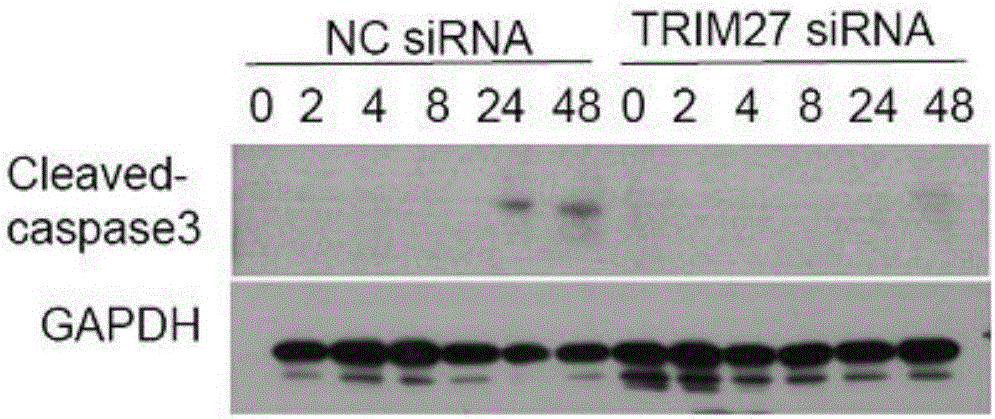
[0022] (A) Experimental method: M. smegmatis was used to infect wild-type and TRIM27 knockout macrophages respectively, and Western blotting was used to detect the changes in the degree of cleavage and activation of the apoptosis marker protein Caspase 3 (Cleaved-caspase 3) at different time points of infection.
[0023] (B) Results: Comparing the protein levels of Cleaved-caspase 3 in wild-type U937 and TRIM27-knockout U937 cells at different infection time points, the results showed that the level of Cleaved-caspase 3 in TRIM27-knockout U937 cells was lower, that is, the effect of TRIM27 on Promotes apoptosis ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com