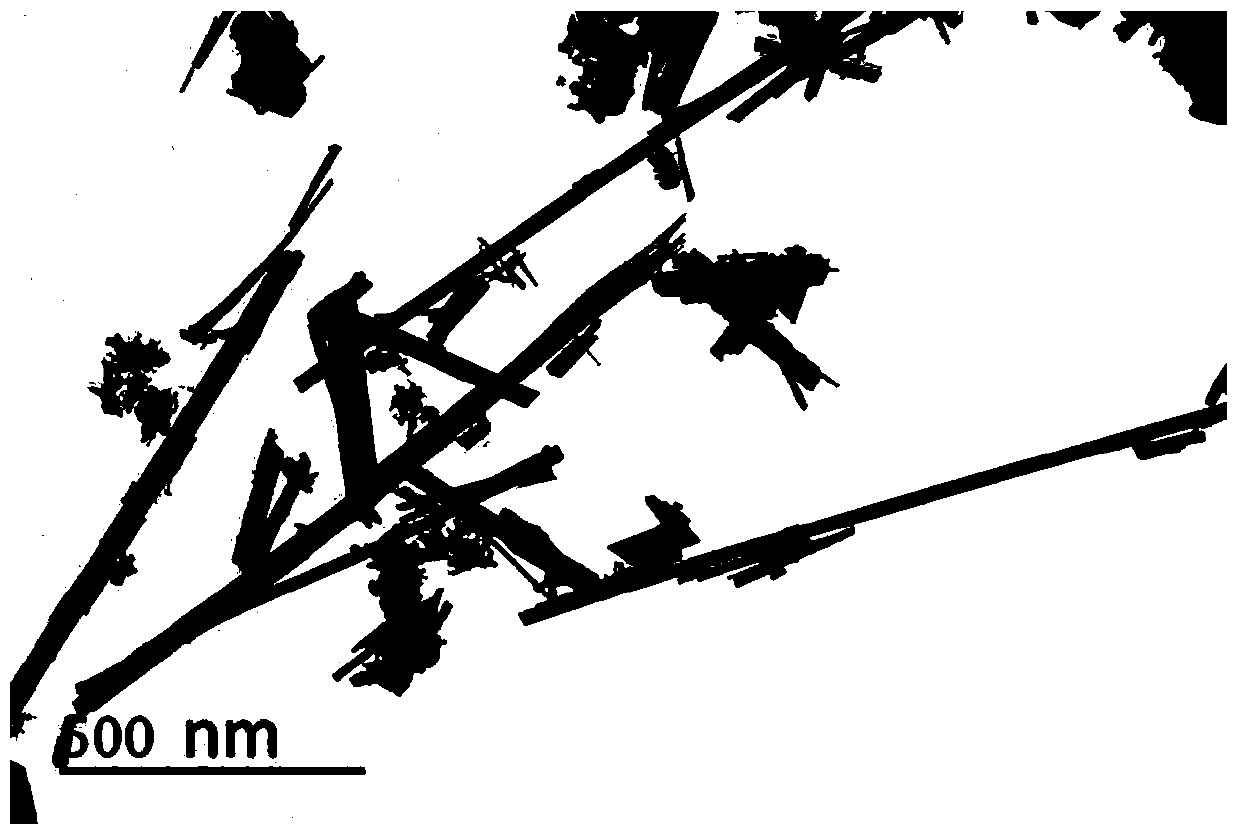
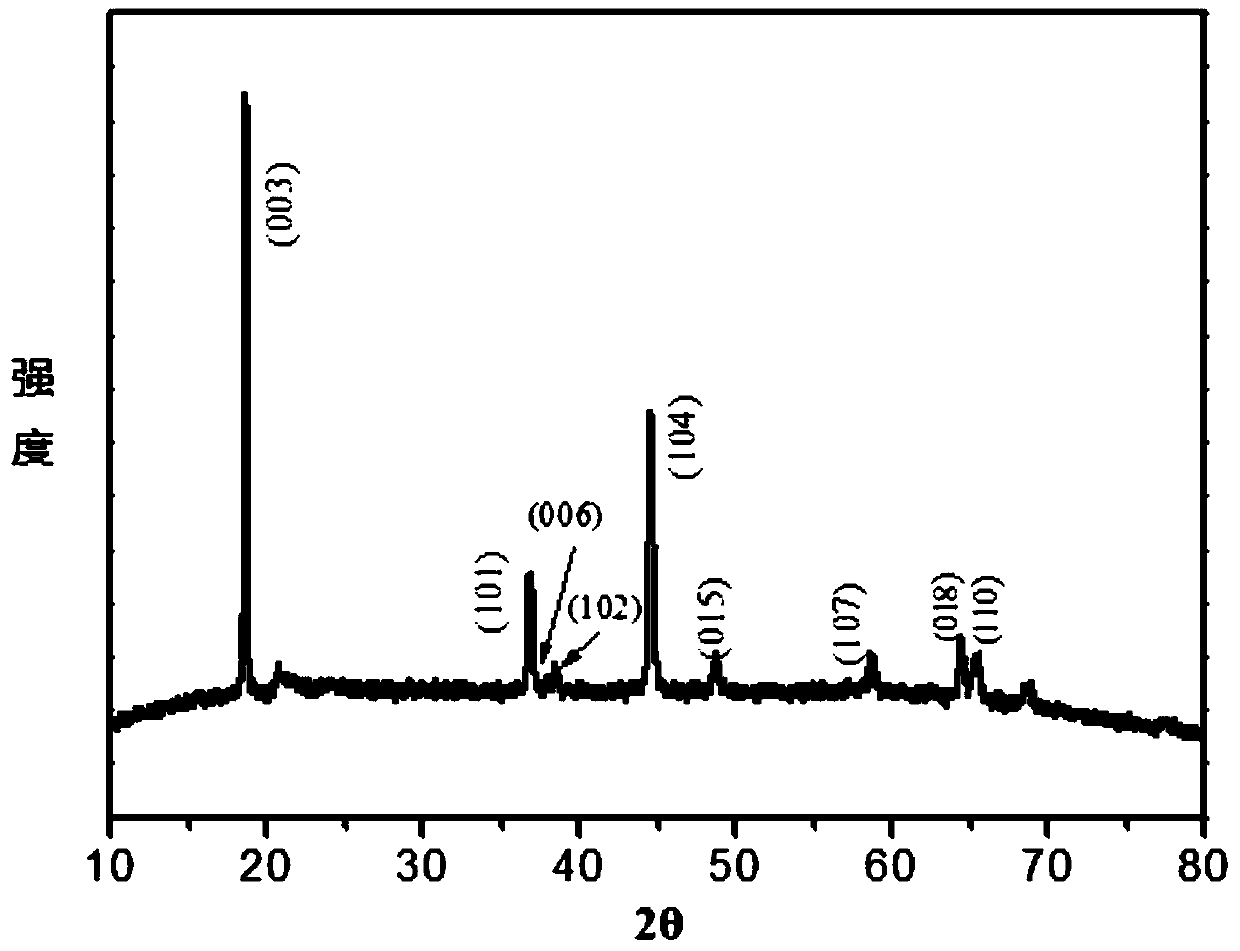
A preparation method of nanowire-like lithium-rich manganese-based positive electrode material
A lithium-rich manganese-based, cathode material technology, applied in battery electrodes, structural parts, electrical components, etc., can solve problems such as poor electronic conductivity and ionic conductivity, large first-round irreversible capacity, and increased irreversible capacity. Achieve the effect of improving electrochemical performance, alleviating the drop of the discharge voltage platform, and facilitating insertion and extraction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) 1.736g MnSO 4 , 3.936g KMnO 4 Soluble in deionized water, configured as Mn 2+ The concentration is 0.4~0.6mol·L -1 (choose 0.5mol L in the present embodiment -1 ), after stirring for 1 hour, transfer the mixed solution into a sealed polytetrafluoroethylene reactor at room temperature, raise the temperature to 160° C., and react for 24 hours.
[0040] After the temperature of the reaction kettle was cooled to room temperature, the precipitate was filtered, and washed alternately with deionized water and absolute ethanol for 3 to 4 times. Dry the washed solid particles at 80°C for 6 hours, and the brown powder after grinding is α-MnO 2 Nanowires.
[0041] 2) soluble 0.09459g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O), 0.09451g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O), 0.1258g lithium hydroxide (LiOH·H 2 O) (5% excess lithium hydroxide) was dissolved in a mixed solution of deionized water and absolute ethanol (the volume ratio was 1:1), and ultrasonically dissolved fo...
Embodiment 2
[0058] 1) soluble 0.08095g cobalt acetate (Co(CH 3 COO) 2 4H 2 O), 0.08087g nickel acetate (Ni(CH 3 COO) 2 4H 2 O), 0.3229g lithium acetate (LiCH 3 COO·2H 2 O) (5% excess lithium acetate) was dissolved in a mixed solution of deionized water and absolute ethanol (the volume ratio was 1:1), and ultrasonically dissolved for 20 minutes to obtain solution A.
[0059] 2) the α-MnO prepared in step 1) in Example 1 2 Nanowires are uniformly dispersed in a mixed solution of ethanol and deionized water (the volume ratio is 1:1) to obtain dispersion B; wherein, the molar ratio of Li, Ni, Co, and Mn metal ions is 1.2:0.13:0.13 :0.54. At room temperature, use a peristaltic pump to add the solution A to the dispersion B dropwise at a constant speed, control the dropping rate to 0.1ml / min, and the rotation speed to 500r / min. After reacting for 4 hours, the cloudy liquid was dried at 80° C. to obtain a dark brown powder.
[0060] 3) Put the dark brown powder obtained in step 2) into...
Embodiment 3
[0062] 1) soluble 0.08095g cobalt acetate (Co(CH 3 COO) 2 4H 2 O), 0.08087g nickel acetate (Ni(CH 3 COO) 2 4H 2 O), 0.3229g lithium acetate (LiCH 3 COO·2H 2 O) (5% excess lithium acetate) was dissolved in a mixed solution of deionized water and absolute ethanol (the volume ratio was 1:1), and ultrasonically dissolved for 20 minutes to obtain solution A.
[0063] 2) the α-MnO prepared in step 1) in Example 1 2 Nanowires are uniformly dispersed in a mixed solution of ethanol and deionized water (the volume ratio is 1:1) to obtain dispersion B; wherein, the molar ratio of Li, Ni, Co, and Mn metal ions is 1.2:0.13:0.13 :0.54. Under the condition of water bath, the temperature is controlled at 60°C, and the solution A is added dropwise to the dispersion B at a constant speed with a peristaltic pump. The dropping rate is controlled to be 0.1ml / min, and the rotation speed is 500r / min. After reacting for 4 hours, the cloudy liquid was dried at 80° C. to obtain a dark brown po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com