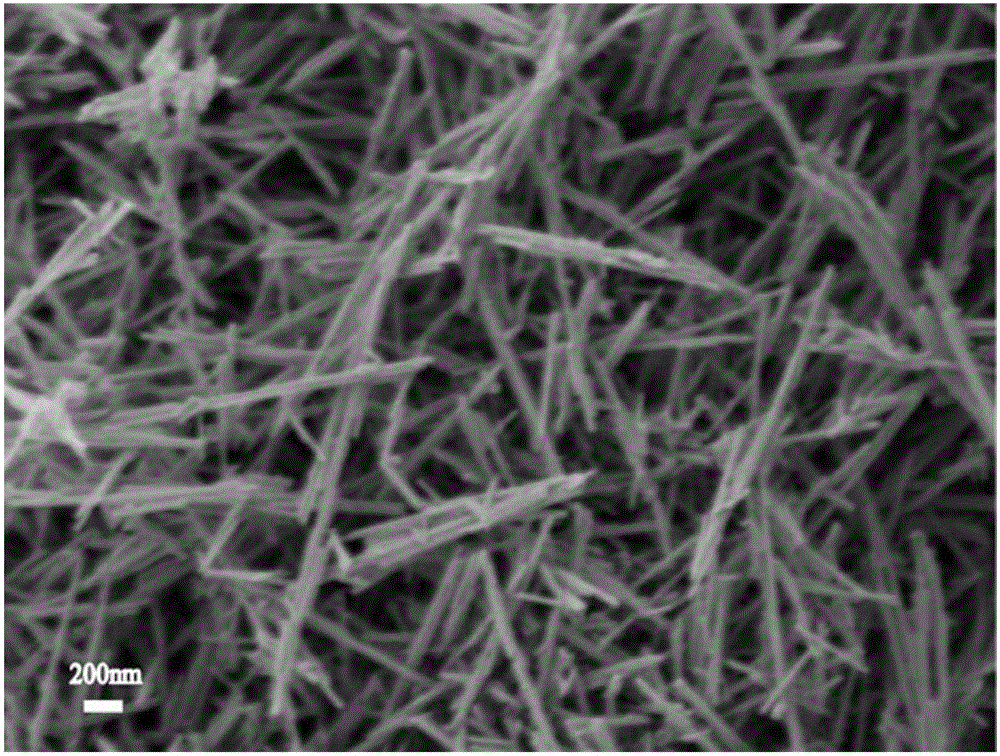
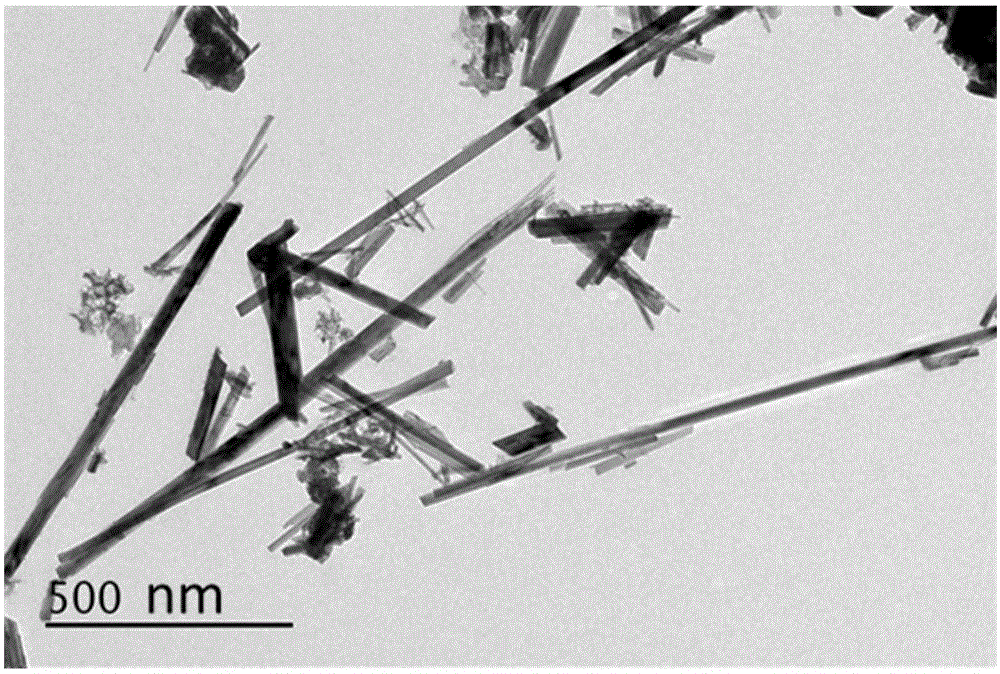
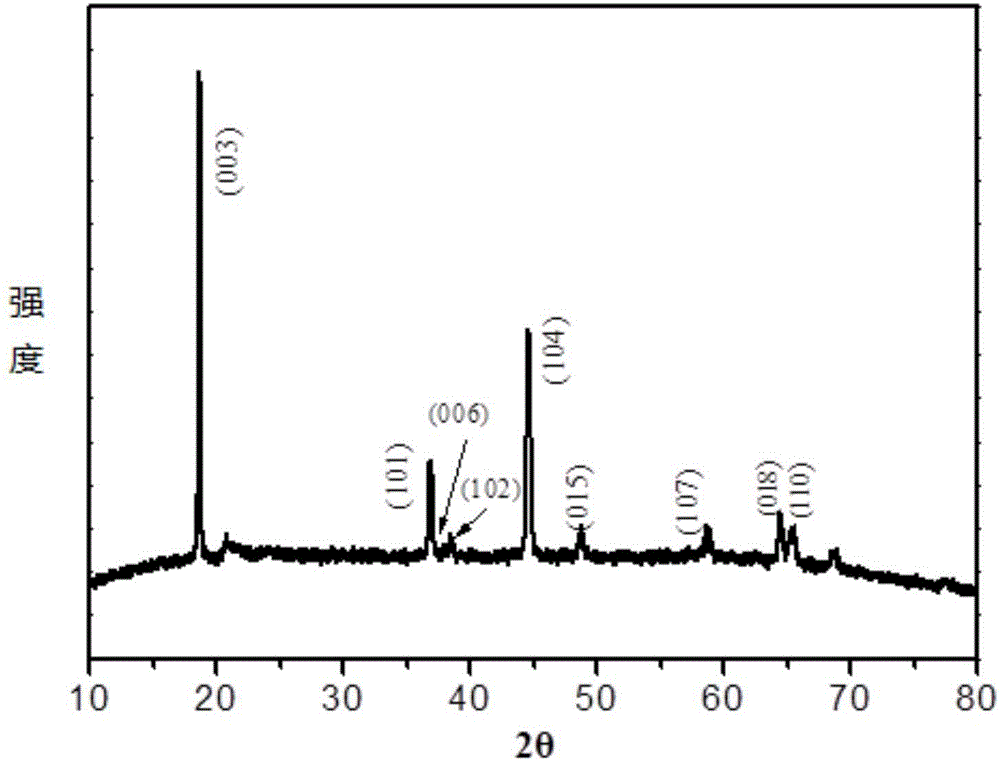
Method for preparing nanowire-shaped lithium-rich manganese-based anode materials
A lithium-rich manganese-based, positive electrode material technology, applied in the direction of battery electrodes, electrical components, electrochemical generators, etc., can solve the problem of poor electronic conductivity and ion conductivity, large irreversible capacity in the first cycle, and increased irreversible capacity, etc. problems, to achieve the effect of improving electrochemical performance, alleviating the drop of discharge voltage plateau, and facilitating intercalation and extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Add 1.736g MnSO 4 , 3.936g KMnO 4 Dissolved in deionized water and configured as Mn 2+ The concentration is 0.4~0.6mol·L -1 (In this example, choose 0.5mol·L -1 After stirring for 1 hour, the mixed solution was transferred to a sealed polytetrafluoroethylene reactor at room temperature, the temperature was raised to 160°C, and the reaction was carried out for 24 hours.
[0040] After the temperature of the reaction kettle is cooled to room temperature, the precipitate is filtered, and then washed alternately with deionized water and absolute ethanol 3 to 4 times. Dry the washed solid particles at 80°C for 6 hours. The brown powder after grinding is α-MnO 2 Nanowires.
[0041] 2) The soluble 0.09459g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O), 0.09451g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O), 0.1258g lithium hydroxide (LiOH·H 2 O) (Lithium hydroxide excess 5%) is dissolved in a mixed solution of deionized water and absolute ethanol (the volume ratio is 1:1), and ultrasonically disso...
Embodiment 2
[0058] 1) The soluble 0.08095g cobalt acetate (Co(CH 3 COO) 2 ·4H 2 O), 0.08087g nickel acetate (Ni(CH 3 COO) 2 ·4H 2 O), 0.3229g lithium acetate (LiCH 3 COO·2H 2 O) (Lithium acetate excess 5%) is dissolved in a mixed solution of deionized water and absolute ethanol (the volume ratio is 1:1), and ultrasonically dissolved for 20 minutes to obtain solution A.
[0059] 2) The α-MnO prepared in step 1) in Example 1 2 The nanowires are uniformly dispersed in a mixed solution of ethanol and deionized water (the volume ratio is 1:1) to obtain dispersion B; wherein the molar ratio of Li, Ni, Co, and Mn metal ions is 1.2:0.13:0.13 :0.54. At room temperature, use a peristaltic pump to drop solution A into dispersion B at a constant speed, control the dropping rate to be 0.1ml / min and the speed to be 500r / min. After 4 hours of reaction, the turbid liquid was dried at 80°C to obtain a dark brown powder.
[0060] 3) Put the dark brown powder obtained in step 2) into a high-temperature tube fur...
Embodiment 3
[0062] 1) The soluble 0.08095g cobalt acetate (Co(CH 3 COO) 2 ·4H 2 O), 0.08087g nickel acetate (Ni(CH 3 COO) 2 ·4H 2 O), 0.3229g lithium acetate (LiCH 3 COO·2H 2 O) (Lithium acetate excess 5%) is dissolved in a mixed solution of deionized water and absolute ethanol (the volume ratio is 1:1), and ultrasonically dissolved for 20 minutes to obtain solution A.
[0063] 2) The α-MnO prepared in step 1) in Example 1 2 The nanowires are uniformly dispersed in a mixed solution of ethanol and deionized water (the volume ratio is 1:1) to obtain dispersion B; wherein the molar ratio of Li, Ni, Co, and Mn metal ions is 1.2:0.13:0.13 :0.54. The temperature is controlled at 60°C under water bath conditions, and solution A is dropped into dispersion B at a constant speed with a peristaltic pump, and the dropping speed is controlled to be 0.1ml / min and the speed is 500r / min. After 4 hours of reaction, the turbid liquid was dried at 80°C to obtain a dark brown powder.
[0064] 3) Put the dark bro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com