A kind of fluorene vinyl derivative and its synthetic method
A synthesis method and derivative technology, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of hydroxyl compounds, etc., can solve the problems of poor optoelectronic properties and poor energy level matching of devices, and achieve the advantages of easy regulation and avoidance of optoelectronic properties. Reduce and avoid the effect of poor solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
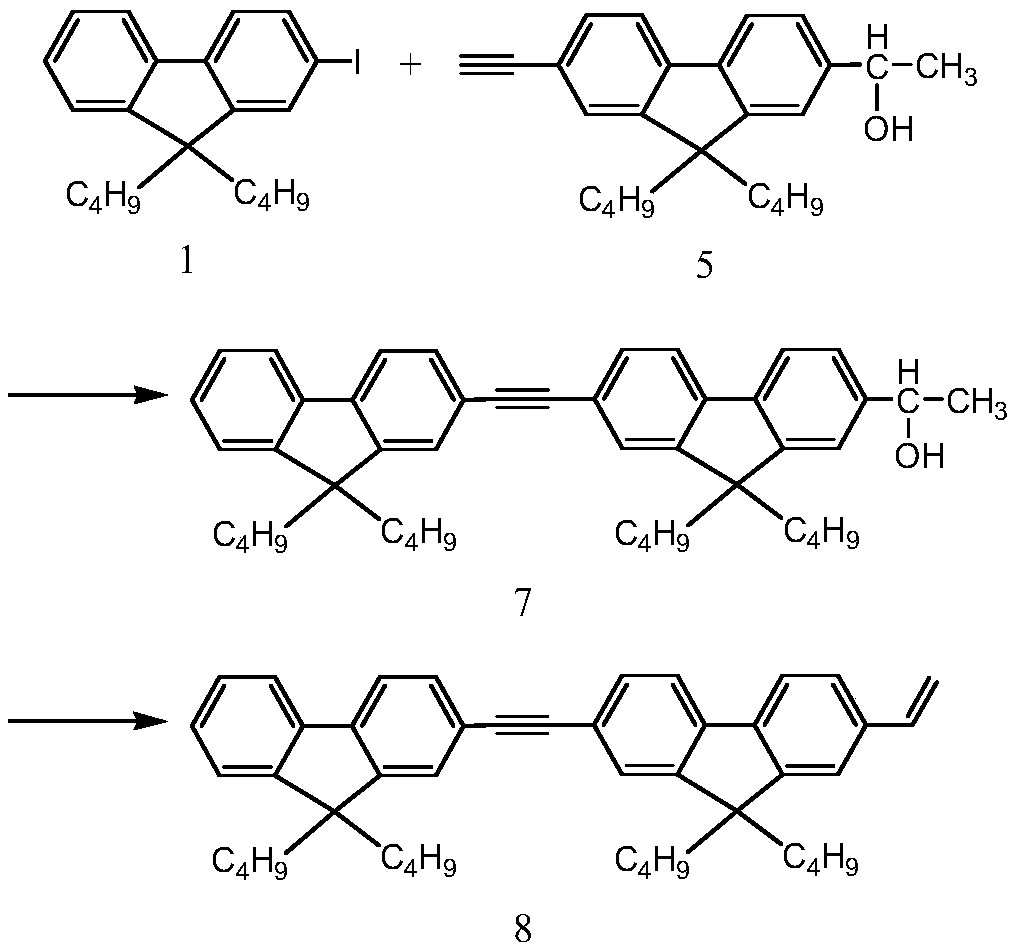
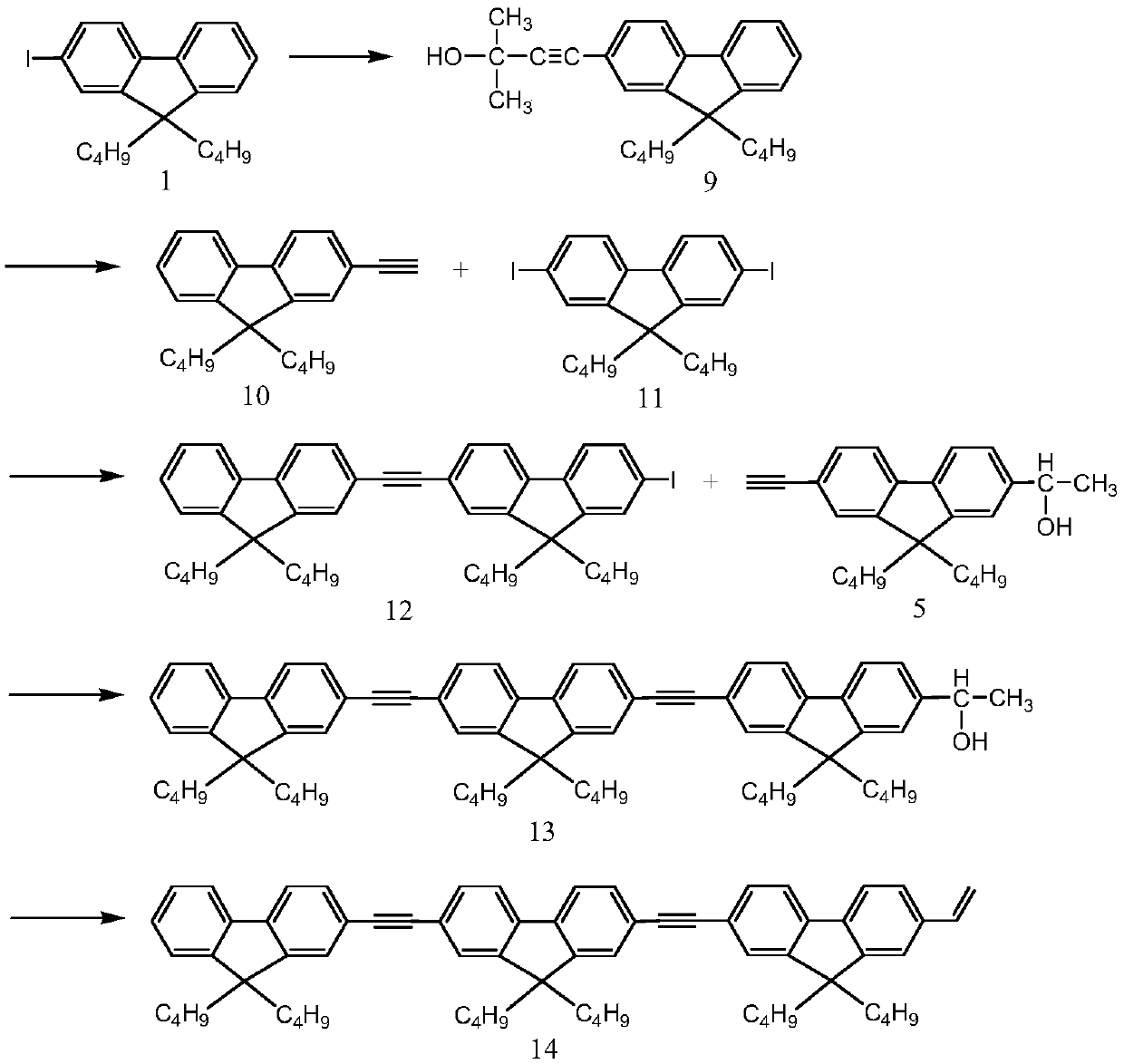
Examples
Embodiment 1
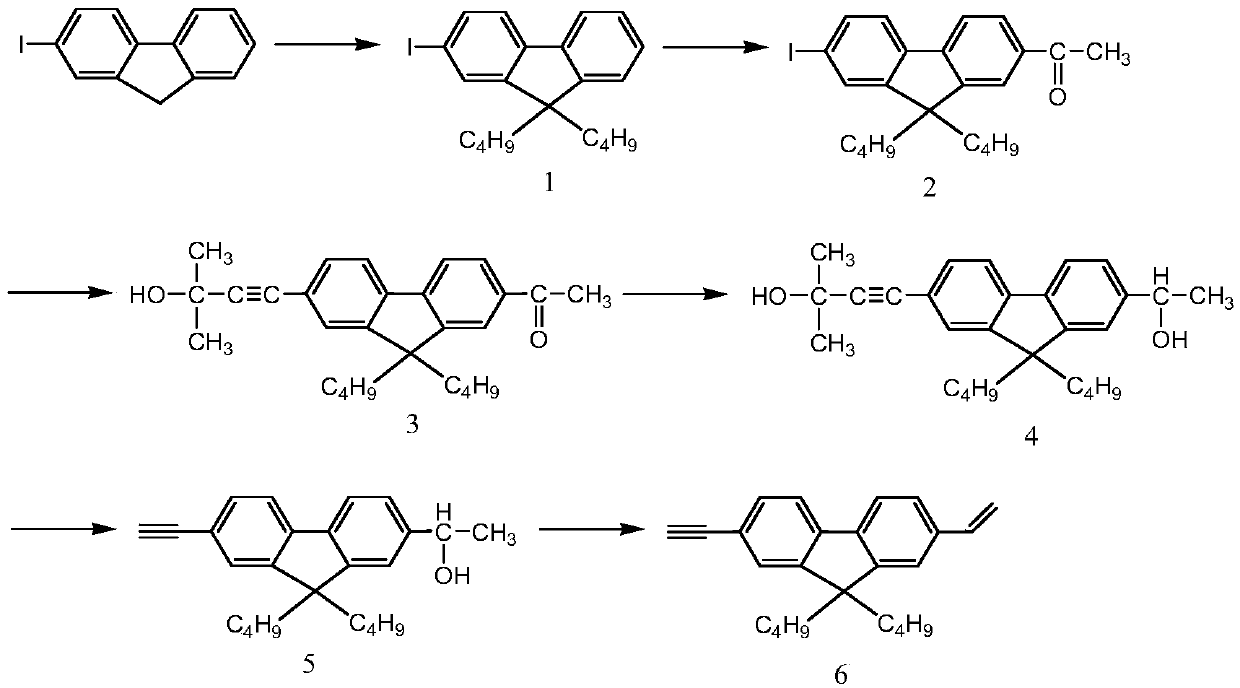
[0047] Example 1: Synthesis of 2-iodo-9,9-dibutylfluorene
[0048] Add 1.46g of 2-iodofluorene, 2ml of n-bromobutane, 0.5ml of DMSO, 0.03g of tetrabutylammonium bromide, and 2ml of 50% NaOH solution in a 25ml round bottom flask. React at 80°C for 6 hours. After the reaction, add 10 milliliters of water and 10 milliliters of petroleum ether, vibrate and separate the liquids, take the upper petroleum ether phase, and extract the water phase with 10 milliliters of petroleum ether once more. Petroleum ether was used as the mobile phase and separated by a chromatographic column. After drying in a vacuum oven at 50°C, the product was a light green solid. Yield 80%. 1 H NMR (ppm) (400MHz CDCl 3 ):7.65-7.24(m,7H).1.95-1.90(m,4H).1.10-1.04(m,4H).0.68-0.54(m,10H).
Embodiment 2
[0049] Example 2: Synthesis of 2-iodo-9,9-dibutylfluoreneacetyl
[0050] Add 0.73 g of anhydrous aluminum trichloride to 20 ml of dichloromethane, stir, and cool in an ice bath for 10 minutes, then slowly drop in acetic anhydride to make the mixture transparent. After sufficient cooling, 1.01 g of 9,9-dibutylfluorene was added in batches and reacted for 12 hours. The mixture was poured into 50 ml of ice water, separated, the aqueous phase was washed twice with 50 ml of dichloromethane, the oil phase was combined, the solvent was spin-dried, and purified by column chromatography to obtain 0.95 g of a light yellow solid. Yield 85%. The eluent is petroleum ether:dichloromethane=5:1. 1 H NMR (ppm) (400MHz CDCl 3 ):7.96-7.94(d,2H).7.74-7.69(m.3H).7.51-7.49(d.1H).2.67(s.3H).2.04-1.92(m.4H).1.10-1.04 (m.4H).0.68-0.65(m.6H).0.57-0.51(m.4H).
Embodiment 3
[0051] Example 3: Synthesis of 2-(2-methyl-3-butyn-2-ol)-9,9-dibutylfluoreneacetyl
[0052] Add 2.23 g of 2-iodo-9,9-dibutylfluoreneacetyl, 2 ml of 2-methyl-3-butynyl-2-ol, 25 ml of tetrahydrofuran, and 5 ml of triethylamine into a 50 ml three-necked flask. After exhausting oxygen with argon, 0.12 g tetrakistriphenylphosphine palladium and 0.06 g cuprous iodide were added. Under the protection of argon, react at 45°C for 6 hours. After the reaction, the mixture was poured into a beaker, 3g of ammonium chloride, 20ml of water and 20ml of dichloromethane were added, the liquid was separated by shaking, and the organic phase was taken and dried with anhydrous magnesium sulfate. Petroleum ether: ethyl acetate 2:1 as mobile phase, column chromatography separation and purification. Yield 80%. 1 H NMR (ppm) (400MHz CDCl 3 ):7.97-7.95(m.2H).7.74-7.68(m.2H).7.44-7.43(d.2H).2.67(s.3H).2.07-1.93(m.4H).1.67(s.6H ).1.11-1.02(m.4H).0.67-0.63(m.6H).0.53-0.47(m.4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com