Chondroitin synthase mutant and application thereof
A technology of chondroitin and mutants, applied in the field of bioengineering, can solve problems such as not being able to meet food and medical safety requirements, and achieve the effects of increased production and large application advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Mutation site selection and mutant library construction of Escherichia coli K4 chondroitin synthase
[0035] By comparing the amino acid sequence of chondroitin synthase derived from Escherichia coli K4 with the amino acid sequence of chondroitin synthase derived from other sources, the following 6 relatively conserved motifs were found, namely L 238 DCDMAP 244 ,D 461 LEVC 465 ,D 231 GS-D 235 , G 516 QLDSDD 522 , P 526 DAVE 530 , N 602 AVDYD 607 . The non-conserved amino acids near these 6 motifs are designed to be mutated at the same time, while the conserved sites remain unchanged, that is, four regions of A are selected 236 -P 246 ,D 461 -D 473 , G 516 -E 530 and I 600 -Y 609 Make combinatorial mutations. Using the "RECODE" mutation strategy, design 4 degenerate primers, in which the stop codon should be avoided when the triplet codon is mutated at the same time, such as using "VNN; V = A, C, G; N = A, T, C, G ", using the constructed pA...
Embodiment 2
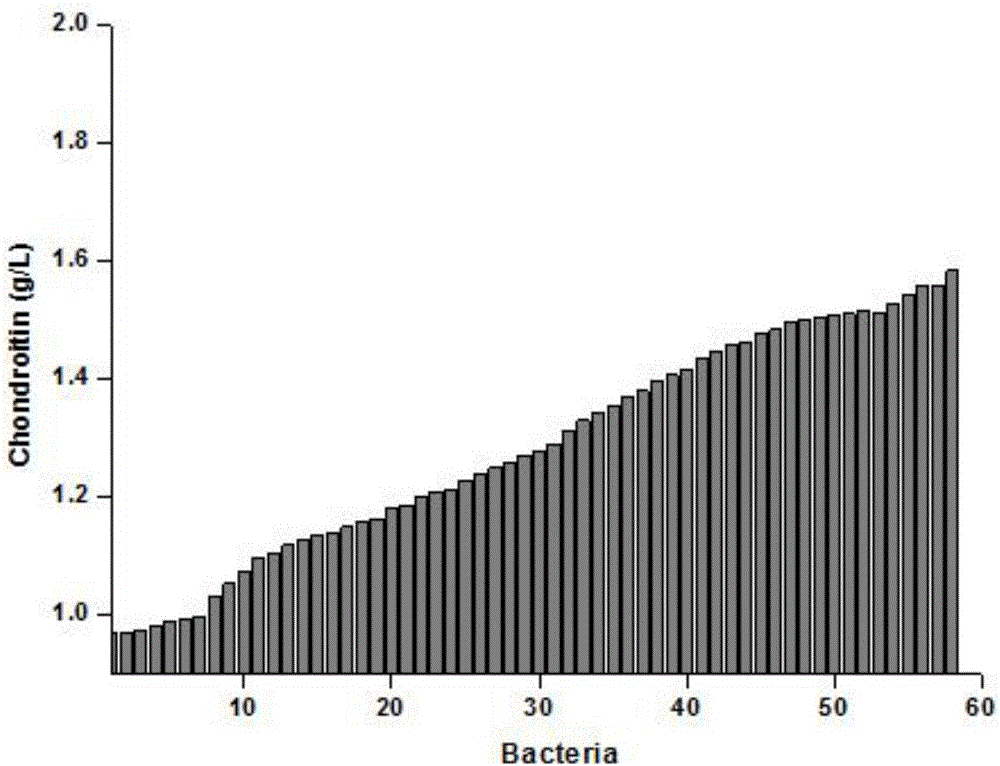
[0049] Example 2 High-throughput screening and shake flask re-screening of recombinant Bacillus subtilis mutant library
[0050] Pick 1000 mutant strains of Bacillus subtilis obtained above, inoculate a single colony in LB medium in a 96-well plate, and culture overnight at 200 rpm at 37°C. Transplant in the fermentation medium of 96 deep-well plate by 10% inoculum amount, and add final concentration 2% xylose to induce in the 2nd hour of fermentation, fermentation medium is: 20g / L yeast powder, 50g / L sucrose, Potassium sulfate 3.9g / L, magnesium sulfate 1.5g / L, 50mM phosphate buffer, pH 7.0. The amount of liquid in each well should not exceed 1 / 3 of the volume of the well, and a well-permeable plate cover should be used to incubate at 200 rpm at 37°C for 48 hours.
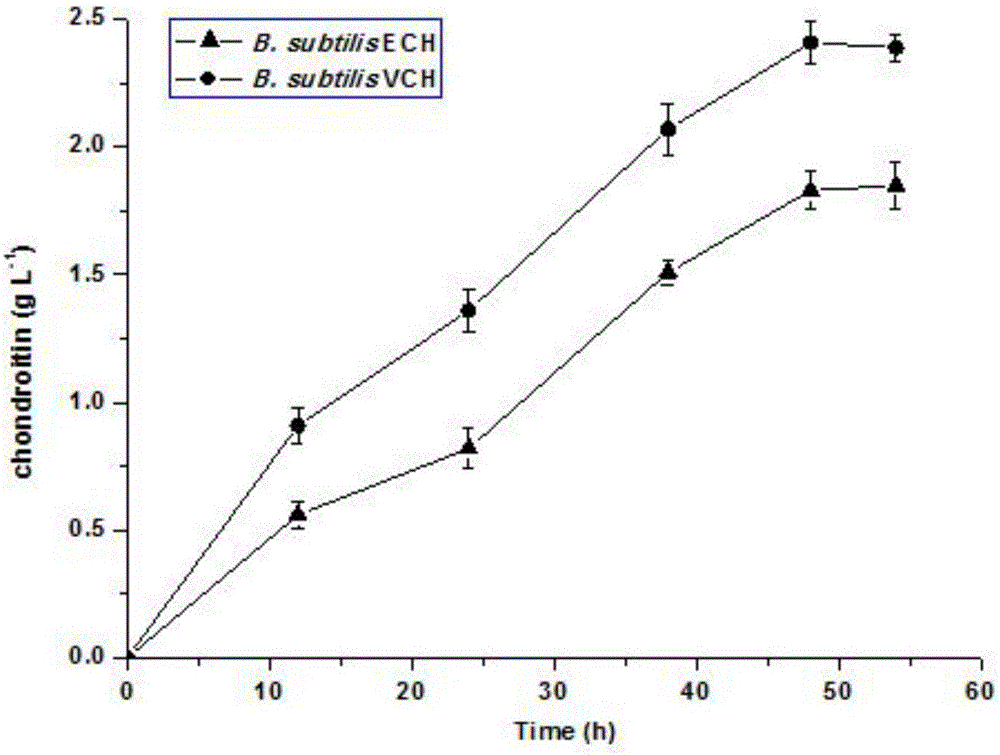
[0051] For the positive mutants obtained by high-throughput screening, shake flask re-screening was carried out, and the original strain B. subtilis ECH was used as a control, and the single clone was inoculated i...
Embodiment 3
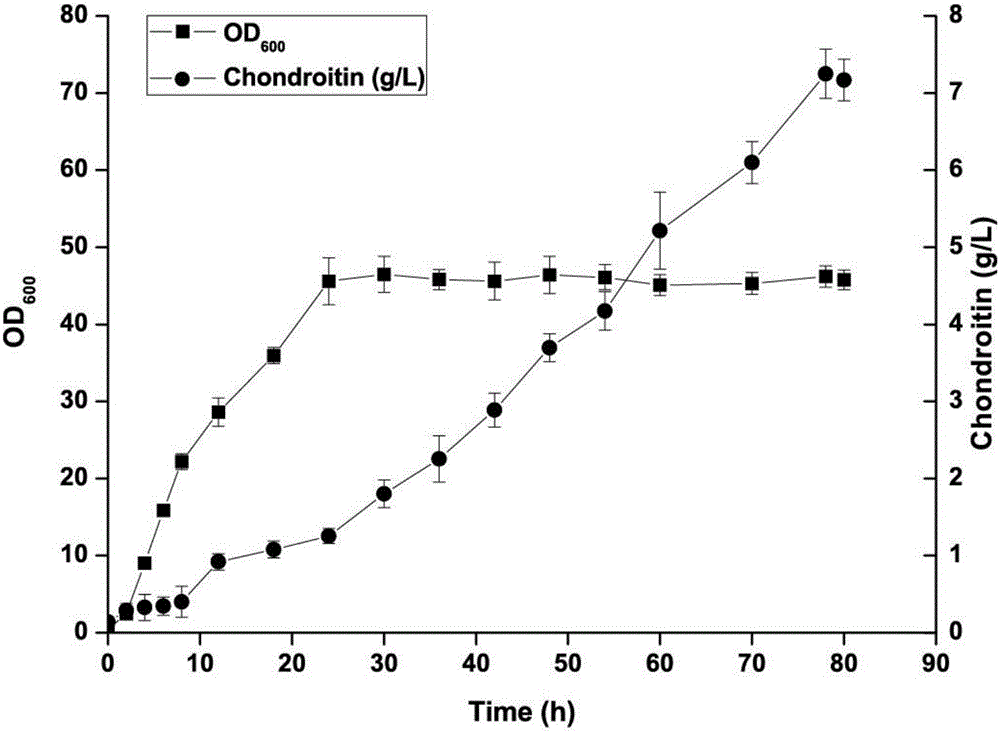
[0056] Embodiment 3 produces the 3L tank fermentation of chondroitin-producing bacillus subtilis mutant strain
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com