A kind of preparation method of telbivudine
A telbivudine and weighing technology is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc. The effect of stable physical and chemical properties, easy operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
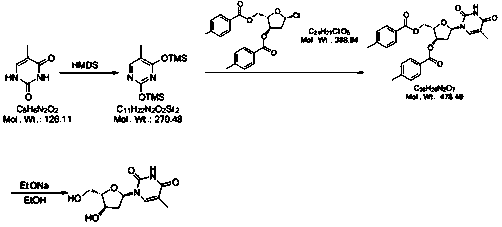
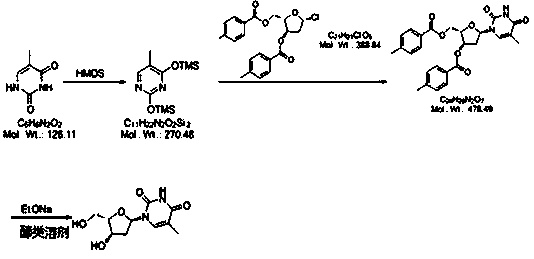
[0043] 1): Weigh 10g thymine, add 50ml dimethyl sulfoxide at room temperature, add 15g hexamethyldisilazane (HMDS), heat to 60-70°C for 28 hours, cool to 0-5°C and store in in the reaction vessel for use.
[0044] 2): Weigh 24g (2S,3R)-5-chloro-2-(((4-methylbenzoyl)oxy)methyl)tetrahydrofuran-3-yl)4-methylbenzoate, Divide into four portions, namely 6g each. Add a portion of the weighed material every 10 minutes, and keep warm at 20-25°C for 12 hours after adding. At the end of the timing, slowly add 200g of saturated NaHCO 3 solution, stirred until the solution was a milky white suspension, and then filtered. The filtrate was concentrated under reduced pressure at 70°C to obtain a viscous mixture containing a large amount of compound B.
[0045]3): Take 120ml of methanol and add it to the concentration container in the previous step, take 1.6g of sodium ethoxide and add it, stir and heat up to 50-60°C for 18 hours, add 40g of cation exchange resin, and keep stirring for 30 ...
experiment example 2
[0048] 1): Weigh 10g thymine, add 70ml dimethylformamide at room temperature, add 18g hexamethyldisilazane (HMDS), heat to 80-90°C for 26 hours, cool to 0-5°C and store in in the reaction vessel for use.
[0049] 2): Weigh 24g (2S,3R)-5-chloro-2-(((4-methylbenzoyl)oxy)methyl)tetrahydrofuran-3-yl)-4-methylbenzoate , are equally divided into four portions, that is, each portion is 6g. Add a portion of the weighed material every 10 minutes, and keep warm at 25-35°C for 10 hours after adding. Slowly add 220g of saturated NaHCO at the end of the timer 3 solution, stirred until the solution was a milky white suspension, and then filtered. The filtrate was concentrated under reduced pressure at 70°C to obtain a viscous mixture containing a large amount of compound B.
[0050] 3): Take 130ml of absolute ethanol and add it to the concentration container in the previous step, take 1.7g of sodium ethoxide and add it, stir and heat up to 65-75°C for 16 hours, add 42g of cation exchang...
experiment example 3
[0053] 1): Weigh 10g thymine, add 80ml N'N-diethylformamide at room temperature, add 19g hexamethyldisilazane (HMDS), heat to 90-100°C for 26 hours, reduce to 0-5 °C and stored in a reaction vessel until use.
[0054] 2): Weigh 24g (2S,3R)-5-chloro-2-(((4-methylbenzoyl)oxy)methyl)tetrahydrofuran-3-yl 4-methylbenzoate, Divide into four portions, ie 6g each. Add one portion of the weighed material every 10 minutes, and keep warm at 35-40°C for 8 hours after adding. Add 230g saturated NaHCO at the end of timing 3 solution, stirred until the solution was a milky white suspension, and then filtered. The filtrate was concentrated under reduced pressure at 70°C to obtain a viscous mixture containing a large amount of compound B.
[0055] 3): Take 140ml of isopropanol and add it to the concentration container in the previous step, take 1.7g of sodium ethoxide and add it, stir and heat up to 75-85°C for 14 hours, add 45g of cation exchange resin, and keep stirring for 50 minutes. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com