Analytical test management system and method
A management system and analyzer technology, applied in the direction of analysis materials, general control system, control/adjustment system, etc., can solve the problems of shortening the time of detection results, lack of training, shortage of personnel, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
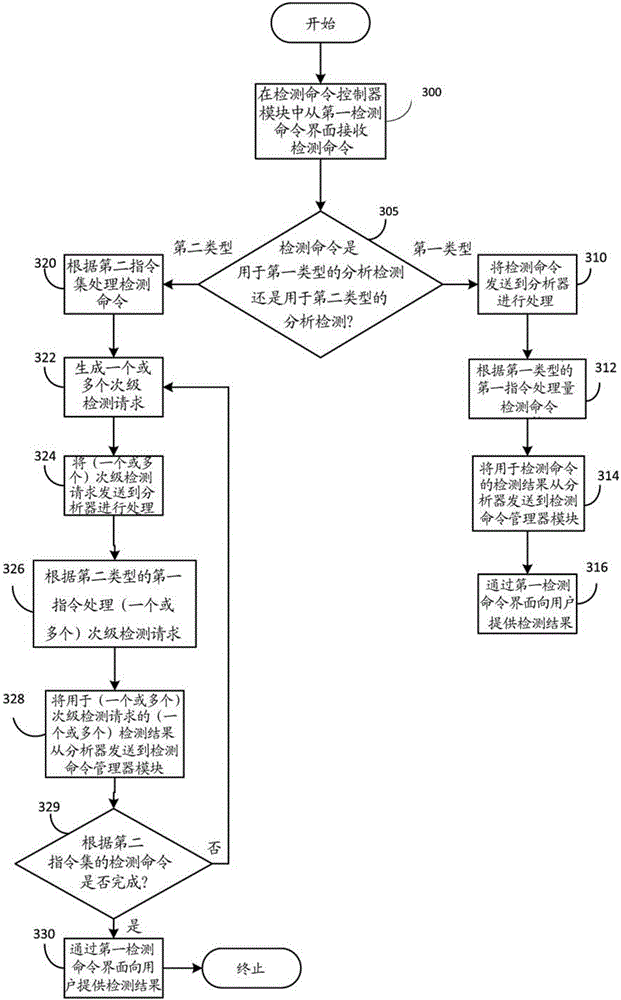
[0059] Example 1: Association of Reagents for a Second Type of Analytical Detection Controlled by the Detection Manager Module
[0060] The order of pipetting in some assays may affect the reliability of some assays. For those experiments, pipetting of other additional reagents may not be possible between the use of two or more specific reagents, and it is desirable that the pipetting steps should be as close in time as possible to each other to yield optimal analytical performance. Such as Figure 4A and Figure 4B As shown, reagents can be linked within one reagent pack (which itself can contain one or more reagents), or between two or more reagent packs (each of which can also contain one or more reagents) are linked between.
[0061] Once connected, a pipetting sequence can be defined on the analyzer, and individual applications of associated assays can then be pipetted for a particular biological sample in a first predetermined sequence. In some embodiments, in case a...
example 2
[0068] Example 2: Restricted pipetting sequence for correlated reagents
[0069] According to the above Figure 4A and Figure 4B The association (either logically or as a set) of embodiments may allow the definition of predetermined pipetting sequences for reagents. In this example, the pipetting sequences for which two specific types of defined pipetting sequences are proposed for association as sets differ in the manner in which the detection process is repeated in the event of a detection failure from a secondary detection request.
[0070] exist Figure 5 In the scheme shown in Reagent Pack A and Reagent Pack B 500, 502 (referred to as semi-associative), the pipetting sequence for detection is defined as the process 504 from Pack A to Pack B. When either or both of the two tests fail, only the failed test needs to be repeated, using either reagent pack A 506 or reagent pack B 508 for the repeat of the test. exist Figure 5In the scheme of , the pipetting order (refer...
example 3
[0071] Example 3: Representative analytical assay of the second type
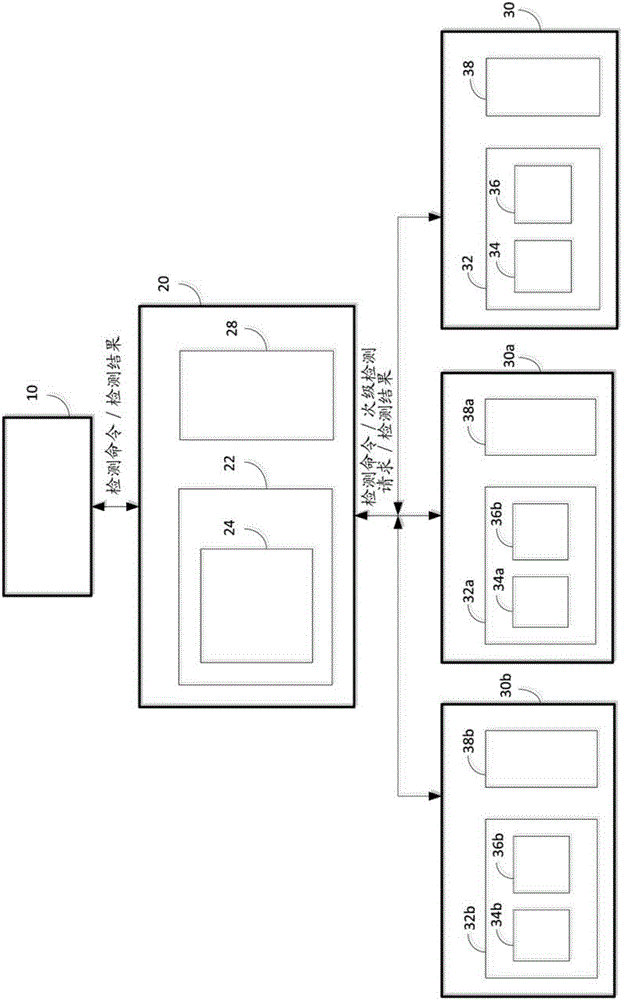
[0072] In this example, representative analysis tests that can be performed under the control of the disclosed test command manager module are described. As in this example and the attached Figure 6 , Figure 7 and Figure 8 As used in , "ACN" refers to the set of instructions that, if present in the detection manager module, represent the second set of instructions, and if present in the analyzer, represent the first set of instructions of the first type (" direct use") or represent the first instruction set of the second type ("restricted").
[0073] Figure 6 The first detection command interface in performing antigen (Ag) / antibody (Ab) dual detection (eg, for HCV, HBV, HIV, Chikungunya, EBV, or the like) is shown in , detection command manager modules, and the interaction between analyzers. In this example, the detection order manager module is shown having the ACNA for Ag / Ab dual detection store...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com