Nucleoside phosphoramidate type compound with HBV/HIV resistance activity and salt and application of nucleoside phosphoramidate type compound
A nucleoside phosphoramidate and compound technology, applied in the field of medicine and chemical industry, can solve the problems of maintaining sufficient concentration of infected parts, poor membrane permeability, and low bioavailability of human body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Reaction formula
[0106]
[0107] (1) Preparation of Compound 2
[0108] Add PMPA (20g, 70mmol), anhydrous acetonitrile (180ml), triethylamine (19.6ml, 140mmol), DMAP (8.52g, 70mmol) and triphenyl phosphite (32.42g, 104mmol) into a three-necked flask, Heat the reaction mixture to 80°C, continue stirring at this temperature for 60 hours until the reaction is complete, distill off most of the solution, add ethyl acetate (100ml) and water (80ml), and wash the aqueous phase with ethyl acetate (2 × 100ml) , the aqueous solution was poured into a reaction flask, adjusted to about pH 3 with concentrated hydrochloric acid (12M, 4.2ml), added 100 mg of seed crystals under stirring at room temperature, and then slowly added concentrated hydrochloric acid (12M, 1.4ml) to adjust to pH 2. Stir at room temperature for 1 hour, then gradually cool to about 10°C, stir overnight, collect the white solid, wash with 20ml (pH 1.5) cold hydrochloric acid solution, and dry to obtain 20.4...
Embodiment 2
[0128] Reaction formula
[0129]
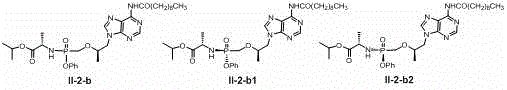
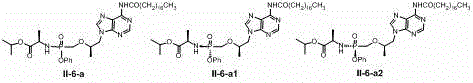
[0130] Add (9-[(R)-2-[[(R)-[[(R)-1(isopropoxy)ethyl]amino]phenoxyphosphoryl]methoxy]propyl] to the reaction vial Adenine) I-1-a1 (120mg, 0.25mmol), triethylamine (70ul, 0.5mmol) and tetrahydrofuran (5ml), under nitrogen protection and 0 ℃, add octanoyl chloride (122mg, 0.75mmol) dropwise, add After completion, stirring was continued overnight at room temperature, the reaction solution was concentrated under reduced pressure, and the residue was separated on a silica gel column (ethyl acetate / hexane, 0-60%) to obtain product II-1-a1 (77 mg, 51%). 1 HNMR (400MHz, CD 3 OD): δ 0.90 (t, 3H), 1.23 – 1.51 (m, 22H), 2.33 (t, 2H), 3.76 (s,1H), 3.84 – 4.21 (m, 4H), 4.27 – 4.47 (m, 1H ), 4.85 – 5.10 (m, 1H), 7.19 –7.57(m, 5H), 8.06 (s, 1H), 8.54 (s, 1H); MS-ESI: 603.4 (M+1) + .
[0131] The following compounds are synthesized in the same way:
[0132] 1 HNMR (400MHz, CD 3 OD): δ 0.89 (t, 3H), 1.23 – 1.51(m, 26H), 2.35 (t, 2H), 3.75 (s, 1H), ...
Embodiment 3
[0144]
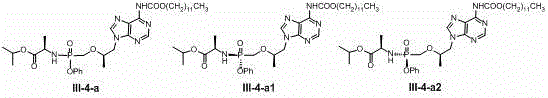
[0145] Add (9-[(R)-2-[[(R)-[[(R)-1(isopropoxy)ethyl]amino]phenoxyphosphoryl]methoxy]propyl] to the reaction vial Adenine) I-1-a1 (120mg, 0.25mmol), N-methylimidazole (41ul, 0.5mmol) and dichloromethane (5ml), under nitrogen protection and 0 ° C, dropwise added hexyl chloroformate ( 135mg, 0.75mmol), the addition was completed, and the stirring was continued overnight at room temperature, the reaction solution was concentrated under reduced pressure, and the residue was separated with a silica gel column (ethyl acetate / hexane, 0-50%) to obtain the product III-1-a1 (96mg , 62%). 1 HNMR (400MHz, CD 3 OD): δ 0.90 (t, 3H), 1.15 – 1.59 (m, 20H), 3.78(s, 1H), 3.82 – 4.22 (m, 6H), 4.27 – 4.48 (m, 1H), 4.84 – 5.11 (m , 1H), 7.16– 7.59 (m, 5H), 8.03 (s, 1H), 8.36 (s, 1H); MS-ESI: 605.4 (M+1) + .
[0146] The following compounds are synthesized in the same way:
[0147] 1 HNMR (400MHz, CD 3 OD): δ 0.92 (t, 3H), 1.16 –1.61 (m, 24H), 3.77 (s, 1H), 3.81 – 4.22 (m, 6H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com