Novel heptacyclic compound and synthesis, activity evaluation and application thereof
A technology of cyclic compounds and drugs, applied in the field of its preparation, can solve problems such as reducing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
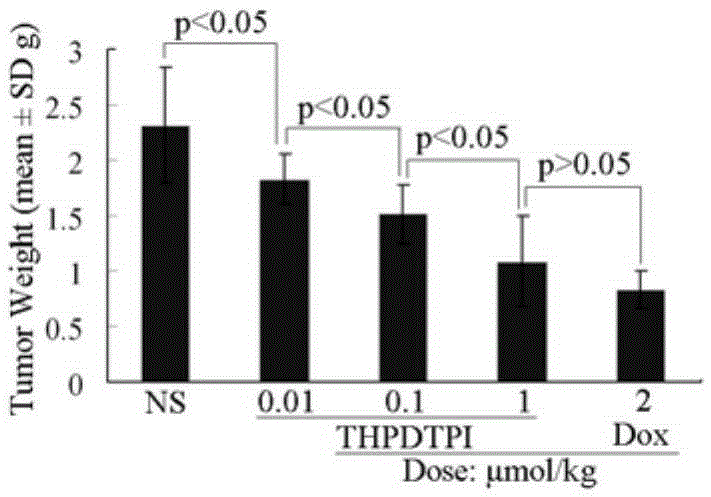
Examples
Embodiment 1
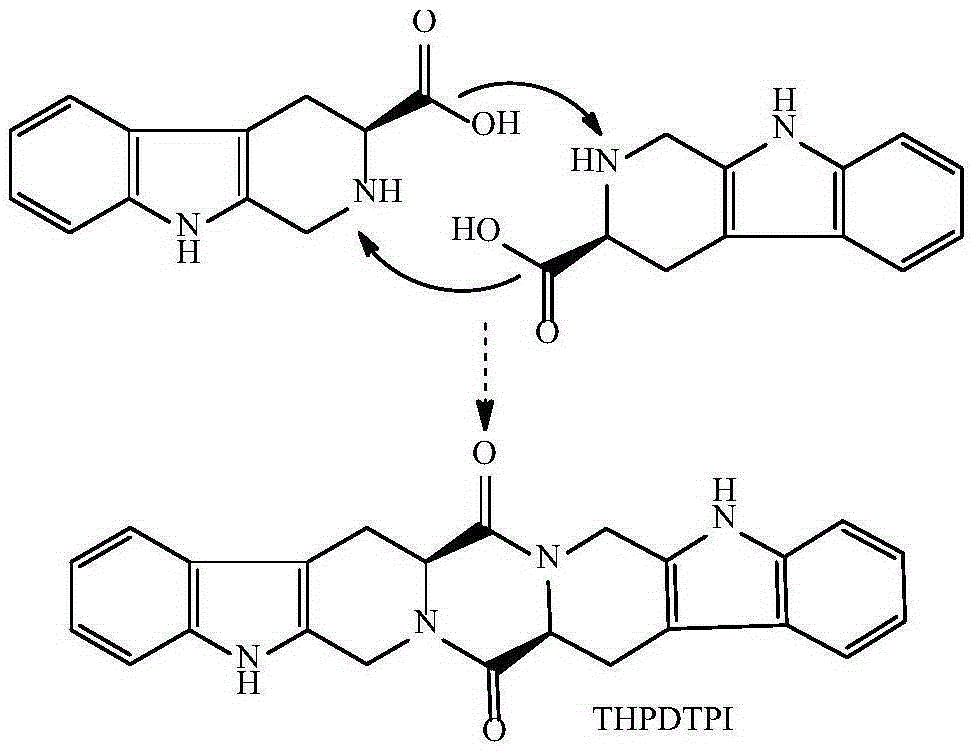
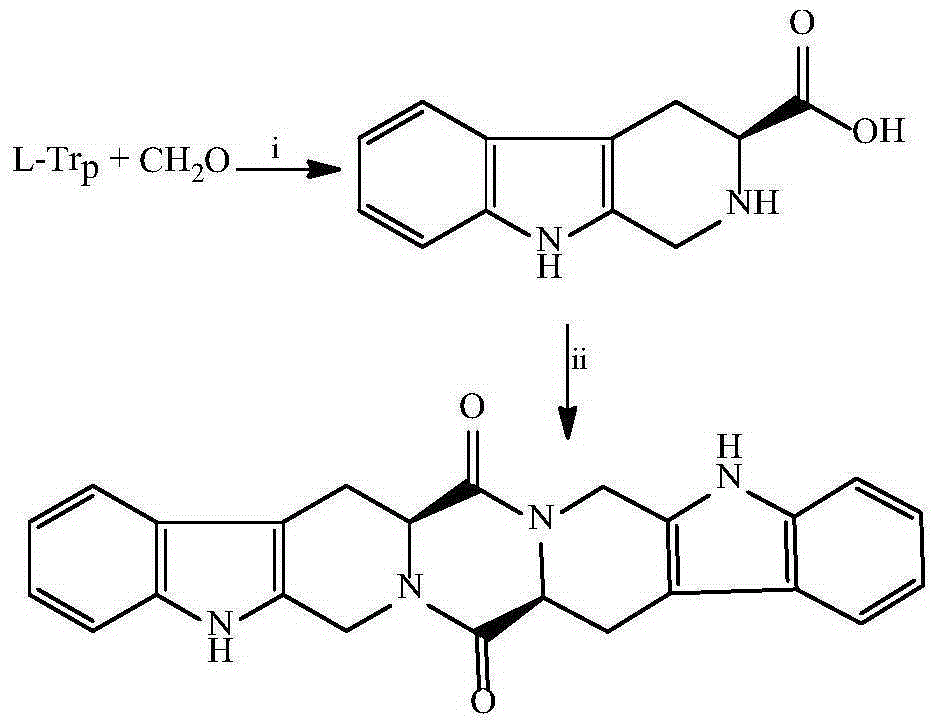
[0019] Example 1 Preparation of 3S-3-carboxy-1,2,3,4-tetrahydro-β-carboline
[0020] To 5.0g (24.5mmol) L-Trp, 25ml H 2 SO 4 Add 8ml formaldehyde (36-38%) to the compound of (1M) and 80ml water. The reaction compound was stirred at room temperature for 2 h, adjusted to pH 7 with concentrated ammonia water, placed at 0°C for 12 h, and the resulting precipitate was filtered out. After recrystallization from acetone, 3.97 g (75%) of the title compound were obtained as colorless crystals. Mp:280-282℃; ESI / MS:217[M+H] + ;IR(KBr):3450,3200,3000,2950,2850,1700,1601,1452,1070,900cm -1 ; 1 HNMR (BHSC-500, DMSO-d6): δ10.99(s, 1H), 9.89(s, 1H), 7.30(t, J=7.5Hz, 1H), 7.22(t, J=8.0Hz, 1H) ,7.01(d,J=8.0Hz,1H),6.81(d,J=7.5Hz,1H),4.01(t,J=4.8Hz,1H),3.75(dd,J=10.5Hz,J=5.0Hz , 1H), 3.64 (dd, J=10.5Hz, J=2.4Hz, 1H), 2.91 (d, J=10.5Hz, 2H), 2.86 (s, 1H).
Embodiment 2
[0021] Embodiment 2 prepares THPDTPI
[0022] Add 0.3mL N -Methylmorpholine to adjust the pH to 9. The reaction mixture was stirred at room temperature for 12 h, TLC (CH 2 Cl 2 / CH 3 OH, 15 / 1) indicated the reaction was complete. The reaction mixture was concentrated under reduced pressure at 45 °C, and the residue was repeatedly triturated with water and acetone, and then purified by silica gel column chromatography (CH 2 Cl 2 / CH 3 OH, 30 / 1), yielding 535 mg (90%) of the title compound as a colorless powder. FT-MS 397.1586[M+H] + . 1 H NMR (800MHz, CDCl 3 ): δ=7.930(s, 2H), 7.448(d, J=8.0Hz, 2H), 7.360(d, J=8.0Hz, 2H), 7.204(t, J=8.0Hz, 2H), 7.124(t ,J=8.0Hz,2H),5.737(d,J=16.0Hz,2H),4.468(dd,J=12.0Hz,J=4.0Hz,2H),4.264(d,J=16.0Hz,2H), 3.535(dd, J=14.4Hz, J=2.4Hz, 2H), 2.927(t, J=13.6Hz, 2H). 13 C NMR (200MHz, CDCl 3 ): δ=169.98, 165.05, 136.71, 136.46, 130.31, 128.40, 126.75, 121.72, 121.60, 119.22, 118.24, 118.15, 111.63, 107.11, 106.01, 57.06, 56.46, 28.06.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com