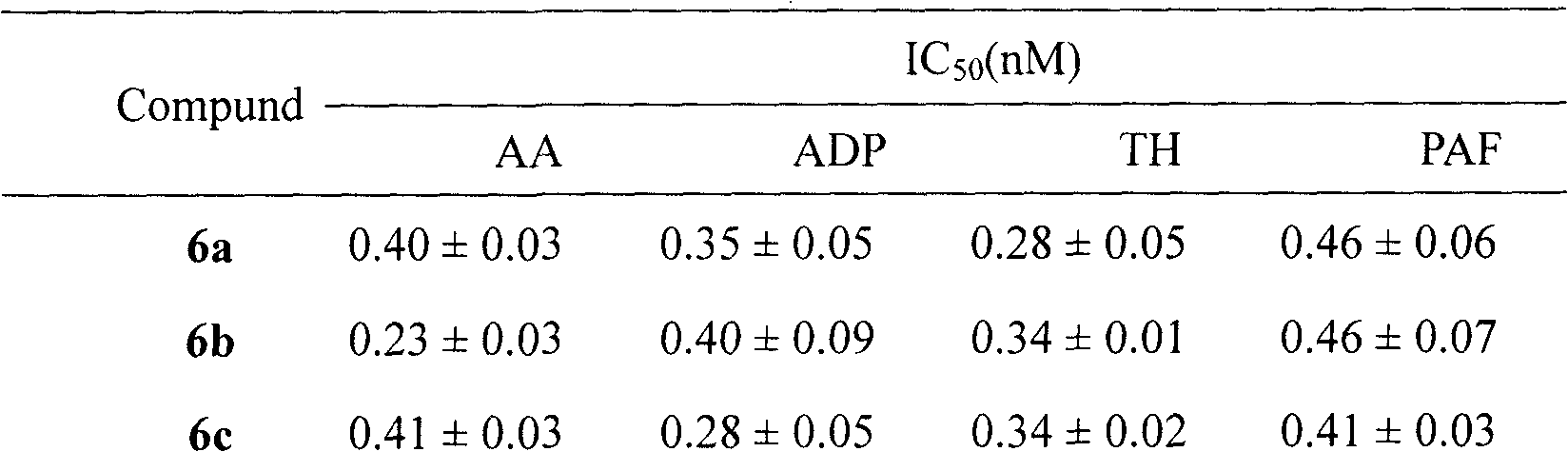
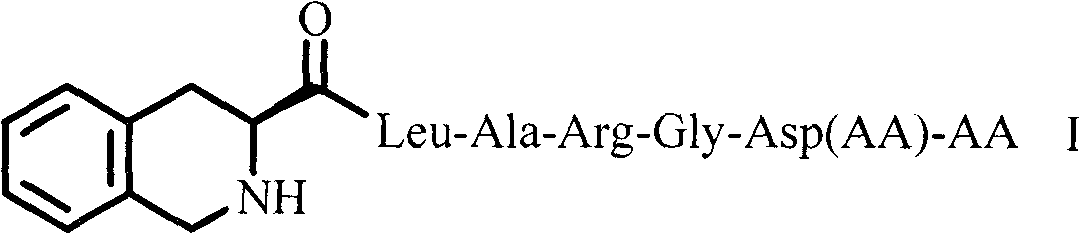Tetrahydroisoquinolinyl-3-carboxylic acid modified LARGD heptapeptides, and synthesis, antithrombotic activity and application thereof
A tetrahydroisoquinoline and antithrombotic technology, applied in the field of biomedicine, can solve problems such as unsatisfactory clinical efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
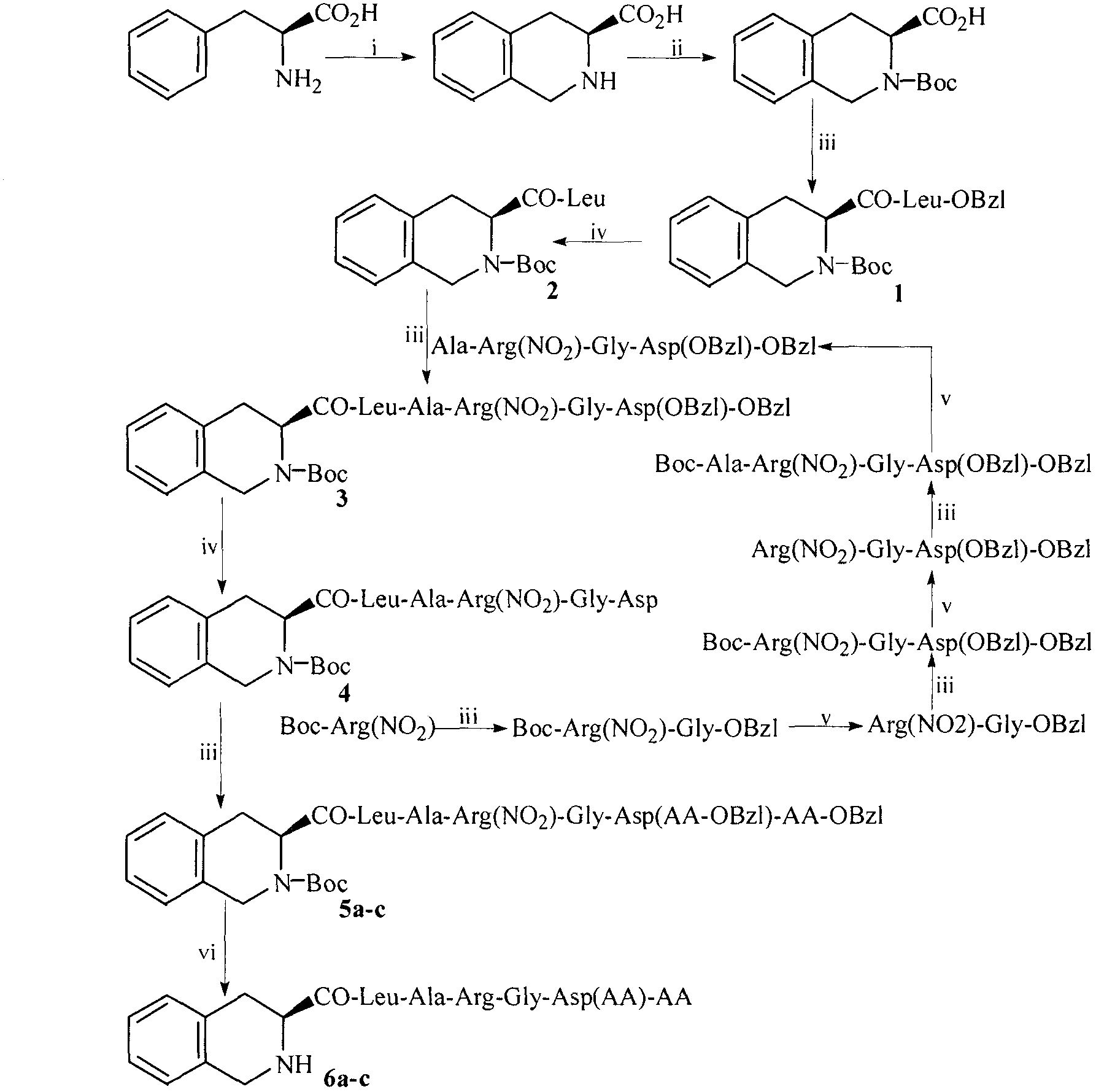
[0025] Example 1 Preparation of (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
[0026] To 4.0g (24.2mmol) L-phenylalanine, first add 21.6ml formaldehyde dropwise, then add 36ml 35% concentrated hydrochloric acid dropwise. Heat the obtained suspension in an oil bath to 95°C and stir for 2 hours to completely dissolve the phenylalanine. After 2.5 hours of reaction, a colorless precipitate begins to form. After 7 hours of reaction, TLC (chloroform / methanol=5 / 1) shows that L-phenylalanine Amino acid disappeared, and 4.2 g of light yellow solid was obtained by suction filtration. Add the obtained pale yellow solid to 86ml of ethanol (80%) and heat in an oil bath at 80°C until the colorless solid dissolves, cool to room temperature, slowly add 2N NaOH solution dropwise, a colorless precipitate precipitates, and when the precipitate reaches the maximum, filter to obtain 4.17 g (97.5%) of the title compound as a colorless solid. ESI-MS(m / e)176[M+H] + .
Embodiment 2
[0027] Example 2 Preparation of (3S)-N-Boc-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
[0028]Dissolve 2.5g (62.2mmol) of sodium hydroxide in 62ml of water under ice bath, then add 10g (56.5mmol) of (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid to prepare a mixture Suspension. 14.8g (67.8mmol) (Boc) dissolved in 40ml tetrahydrofuran 2 O was added to the suspension. The reaction mixture was stirred for 24 h, and the CO produced by the reaction was continuously removed during the reaction. 2 , when the solution became clear, TLC (methanol / chloroform: 1:10) showed that (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid disappeared, and the reaction was stopped. The reaction mixture was concentrated under reduced pressure to remove tetrahydrofuran, and the resulting oil was dissolved in ethyl acetate. The resulting solution was sequentially washed with 5% KHSO 4 Wash with saturated NaCl aqueous solution. The organic layer was dried over anhydrous sodium sulf...
Embodiment 3
[0029] Example 3 Preparation of N-[(3S)-N-Boc-1,2,3,4-tetrahydroisoquinoline-3-formyl]-Leu-OBzl
[0030] Add 149mg (1.1mmol) N -Hydroxybenzotriazole (HOBt), after stirring for 10 min, 221 mg (1.1 mmol) of dicyclohexylcarbodiimide (DCC) was added to obtain the reaction solution (I). Suspend 393 mg (1 mmol) of Tos·Leu-OBzl in 4 ml of anhydrous THF, add 1 ml of N-methylmorpholine (NMM) to adjust the pH to 9, and stir to obtain the reaction solution (II). Add the reaction solution (I) to the reaction solution (II), stir at room temperature for 12h, TLC (ethyl acetate / petroleum ether, 1:3) shows that (3S)-N-Boc-1,2,3,4-tetrahydro The isoquinoline-3-carboxylic acid disappeared. Dicyclohexylurea (DCU) was filtered off, the filtrate was concentrated to dryness under reduced pressure, and the residue was dissolved in 50 ml of ethyl acetate. The resulting solution was sequentially washed with 5% NaHCO 3 Wash 3 times with aqueous solution, 3 times with saturated NaCl aqueous solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com