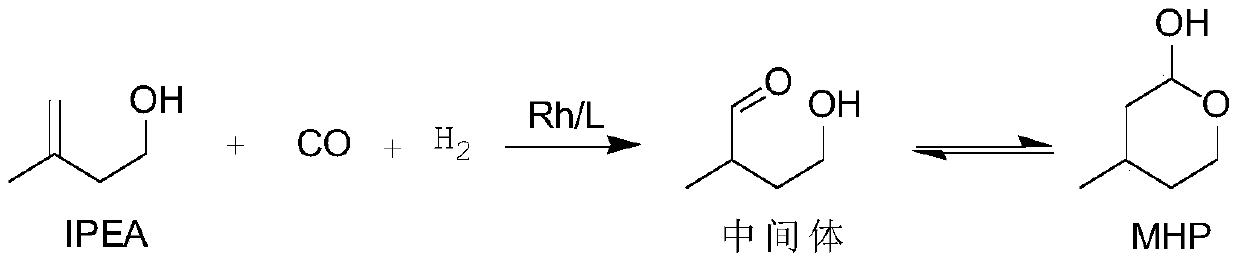
A kind of method for preparing 2-hydroxyl-4-methyltetrahydropyran
A methyltetrahydropyran, hydroxyl technology, applied in the direction of carbon monoxide reaction preparation, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem that rhodium catalysts are difficult to recycle, rhodium catalysts and phosphine Difficult to recycle ligands, catalyst loss and other problems, to achieve the effect of inhibiting acetal side reactions, simple and reliable process route, and improving reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
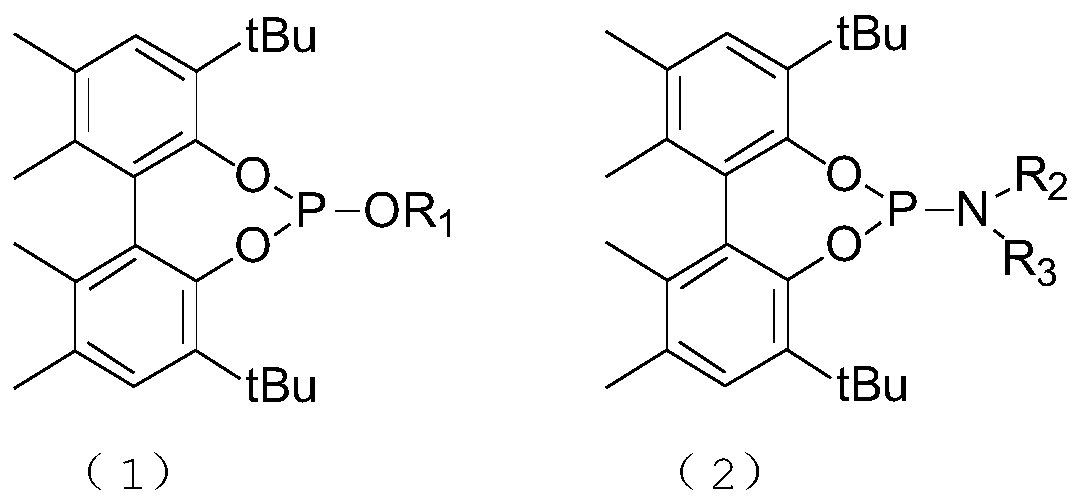
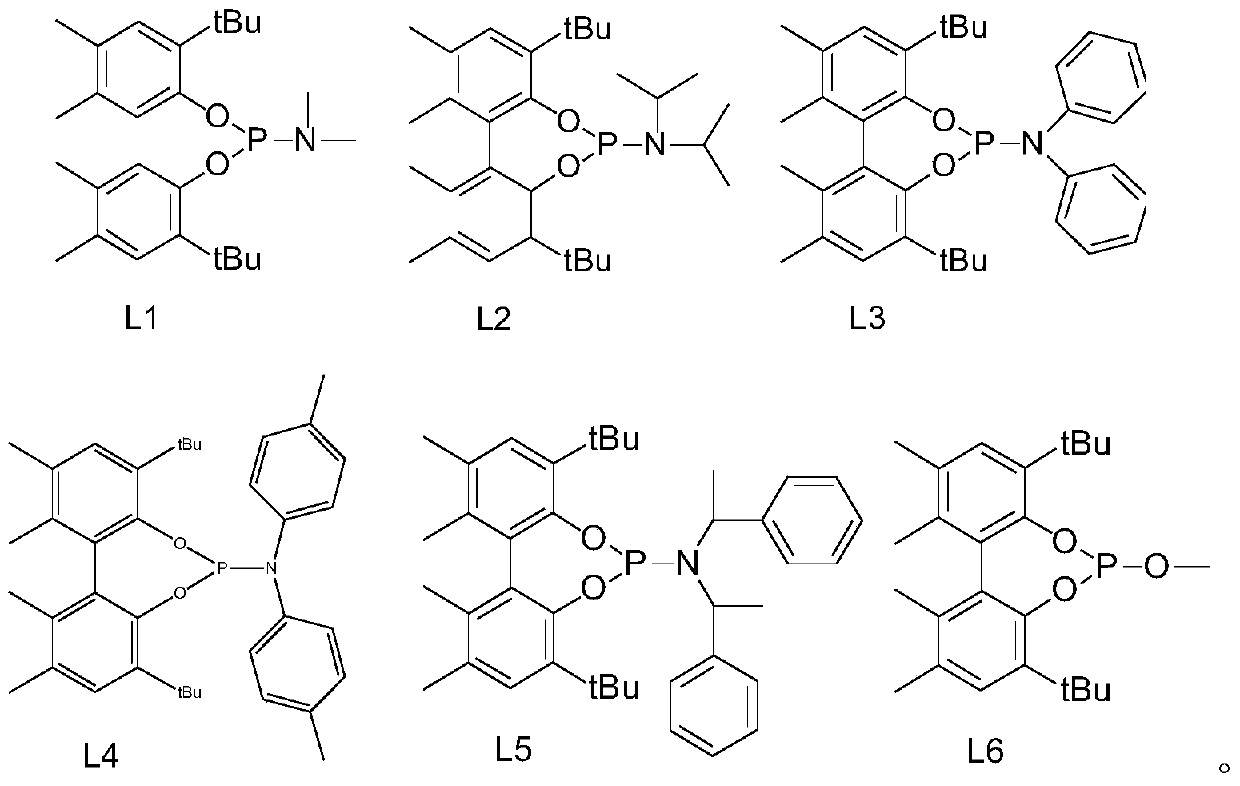
[0061] Ligand L1 is 4,8-di(1,1-tert-butyl)-N,N,1,2,10,11-hexamethyl-dibenzo[d,f][1,3,2] Synthesis of Dioxaphosphorin-6-amine
[0062] a) Synthesis of 3,4-dimethyl-6-tert-butylphenol
[0063] Preheat 122.0g of 3,4-dimethylphenol to 65°C, add 2.5ml of concentrated sulfuric acid, feed isobutene at a flow rate of 0.2L / min, keep the reaction at 70°C for 2h, add 20wt% sodium hydroxide aqueous solution to neutralize the reaction solution , separated and retained the organic phase, and distilled under reduced pressure to obtain 114.1 g of 3,4-dimethyl-6-tert-butylphenol.
[0064] b) Synthesis of 3,3'-di-tert-butyl-5,5',6,6'-tetramethyl-2,2'-dihydroxybiphenyl
[0065] Dissolve 82g of 3,4-dimethyl-6-tert-butylphenol and 5g of potassium chromate in 300ml of acetic acid, heat to 90°C, and inject oxygen for 5h. Oxygen was released after the reaction, acetic acid was removed by atmospheric distillation, then 200ml of deionized water was added, and 46.9g of solid was obtained by filtratio...
Embodiment 2
[0069] Ligand L2 is 4,8-bis(1,1-tert-butyl)-1,2,10,11-tetramethyl-N,N-bis(1-methylethyl)-dibenzo[d ,f][1,3,2]Synthesis of dioxaphosphorin-6-amine
[0070] Dissolve 3.4g of phosphorus chloride in 40ml of ether, add 2.5g of diisopropylamine dropwise, stir at room temperature for 0.5h, raise the temperature to 100°C for 3h, release hydrogen chloride gas, and obtain dichlorodiisopropylaminophosphine ether solution. Dissolve 8.2g of 3,3'-di-tert-butyl-5,5',6,6'-tetramethyl-2,2'-dihydroxybiphenyl and 2g of pyridine in 50ml of ether, stir and heat to reflux, slowly Dichlorodiisopropylaminophosphine ethyl ether solution was added dropwise, and the addition was completed in 1h. Cool down to room temperature, filter, diethyl ether is removed from the filtrate by vacuum distillation, 100ml of deionized water is added to the residue, solids are precipitated, the filter cake is washed with deionized water after filtration, and 6.5g of solids are obtained after drying, namely 4,8- Bis(1,1...
Embodiment 3
[0072] Ligand L3 is 4,8-di(1,1-tert-butyl)-1,2,10,11-tetramethyl-N,N-diphenyl-dibenzo[d,f][1, 3,2] Synthesis of dioxaphosphorin-6-amine
[0073]Dissolve 3.4g of phosphorus chloride in 40ml of diethyl ether, add 4.2g of diphenylamine dropwise, stir at room temperature for 0.5h, raise the temperature to 100°C for 3h, and obtain dichlorodiphenylaminophosphine ether solution. Dissolve 8.2g of 3,3'-di-tert-butyl-5,5',6,6'-tetramethyl-2,2'-dihydroxybiphenyl and 2g of pyridine in 50ml of ether, stir and heat to reflux, slowly Dichlorodiphenylaminophosphine ethyl ether solution was added dropwise, and the addition was completed in 1h. Lowered to room temperature, filtered, the filtrate was distilled under reduced pressure to remove ether, 100ml of deionized water was added to the residue, and solids were precipitated. After filtration, the filter cake was washed with deionized water and dried to obtain 6.9g of solids, namely 4,8- Bis(1,1-tert-butyl)-1,2,10,11-tetramethyl-N,N-dipheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com