Methods for producing recombinant glycoproteins with modified glycosylation
A glycoprotein and glycosylation technology, applied in biochemical equipment and methods, glycosylase, chemical instruments and methods, etc., can solve the problem of high cost of recombinant protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0087] method
[0088] i. Construction of expression vectors for production of fucosidase or endoglycosidase
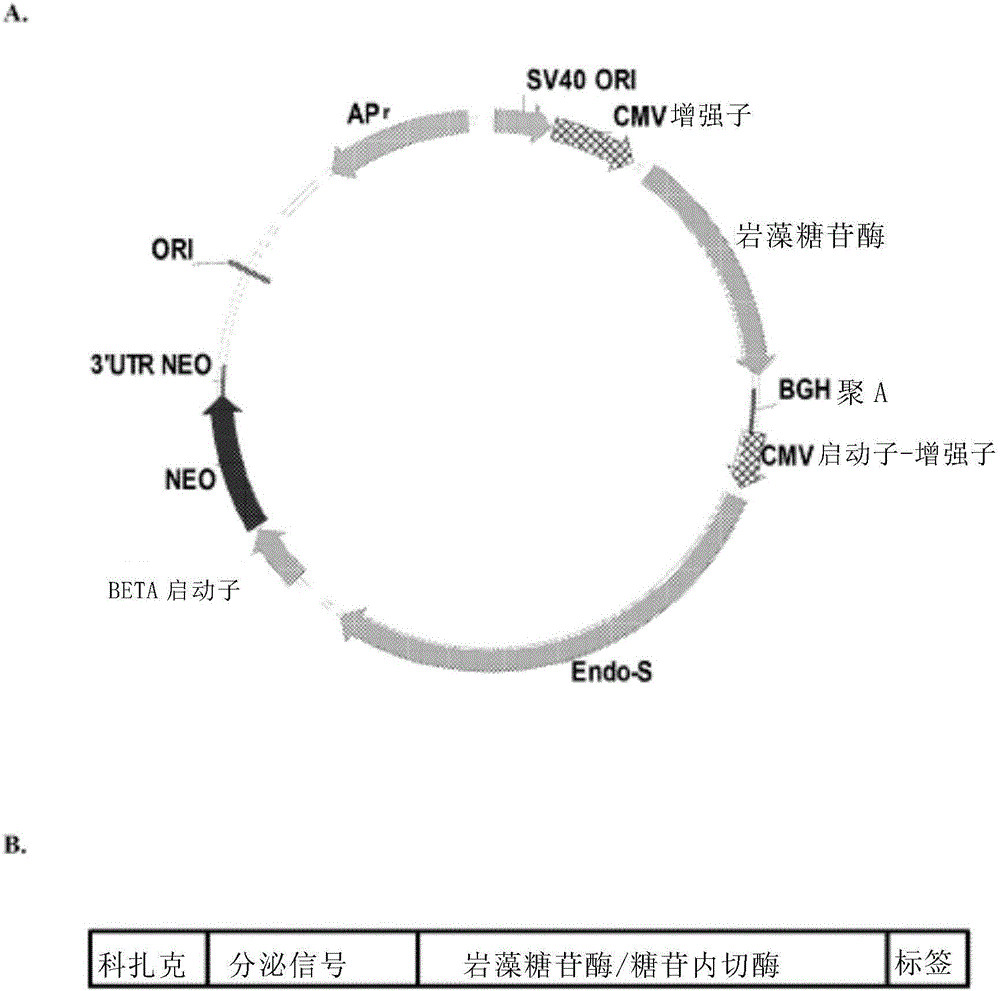
[0089] In order to construct the expression vector of fucosidase or endoglycosidase, the fucosidase or endoglycosidase gene was isolated by conventional techniques, and codon optimization was performed based on the codon usage of hamster cells. The synthetic gene was prepared by GeneArt Corp, and inserted into the pcDNA3.1B(-)Myc-His vector (Invitrogen, US) at the restriction enzyme site Bgl II / EcoR I ( figure 2 Middle A). The expression cassette for α-fucosidase, from 5' to 3', comprises Kozak sequence, Igk leader sequence, fucosidase coding sequence and His-tag coding sequence. figure 2 Middle B. The endoglycosidase expression cassette, from 5' to 3', comprises a Kozak sequence, an Igk leader sequence, an endoglycosidase coding sequence, and a His-tag coding sequence. figure 2 Middle B.
[0090] ii. Preparation of afucosylated antibody
[0091] Antibody-pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com