A kind of 8-amino-4,6-diyn-1-alcohol compound and its preparation method and application
A technology of compounds and alcohols, which is applied in the field of 8-amino-4,6-diyn-1-ols and their preparation, and can solve problems such as easy recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
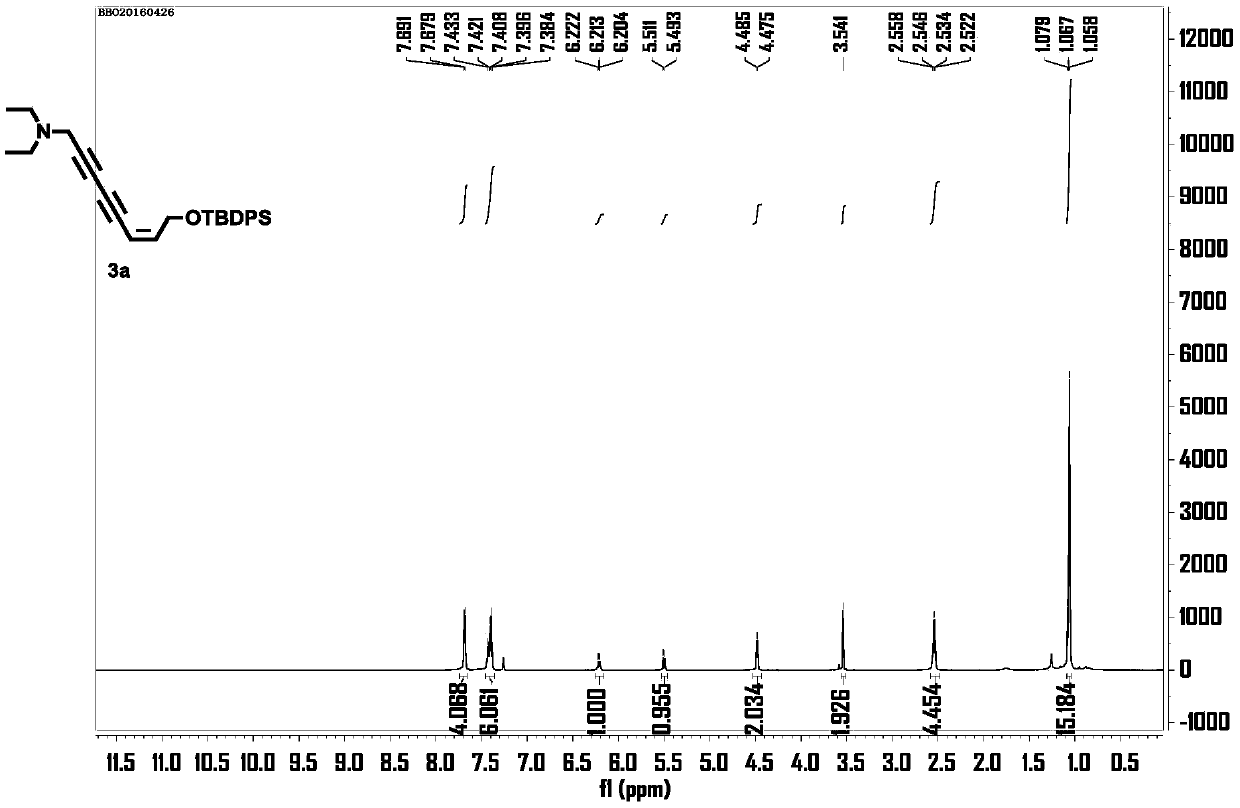
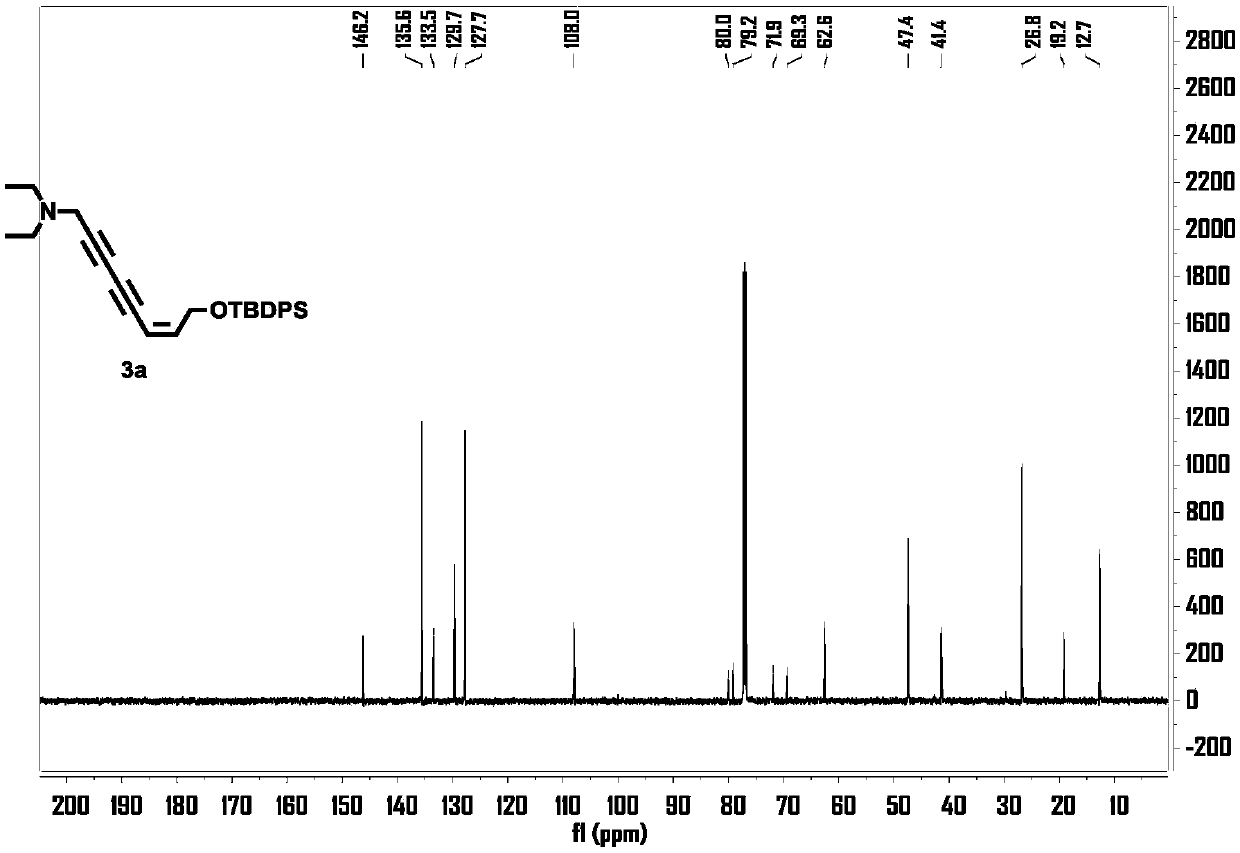
[0022] 1, the preparation of (Z)-8-((tert-butyldiphenylsilyl)oxy)-N,N-diethyl-6-octene-2,4-diyn-1-amine ( 3a)
[0023] Take a 10mL round-bottomed flask, dissolve 22.5μL (0.16mmol) of 3-diethylamino-1 propyne and 5.9mg (0.03mmol) of cuprous iodide in 0.6mL of piperidine under the protection of argon, and place in an ice-water bath Cool to 0°C, slowly drop (Z)-(5-bromo-2-penten-4-yne-1-oxyl)tert-butyldiphenylsilane 50mg (0.13mmol) in 0.2 mL of the solution formed by piperidine, after the addition, slowly warm up to room temperature, react at room temperature for 4h, add 0.5mL of saturated ammonium chloride solution to terminate the reaction, then extract with ethyl acetate (0.3mL x 2), combine organic phase, washed with saturated NaCl solution, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure, using V (petroleum ether): V (ethyl acetate) = 2:1 as the eluent, silica gel column chromatography to obtain light yellow ...
Embodiment 2
[0040] Corticosterone-injured PC12 cell protection experiment:
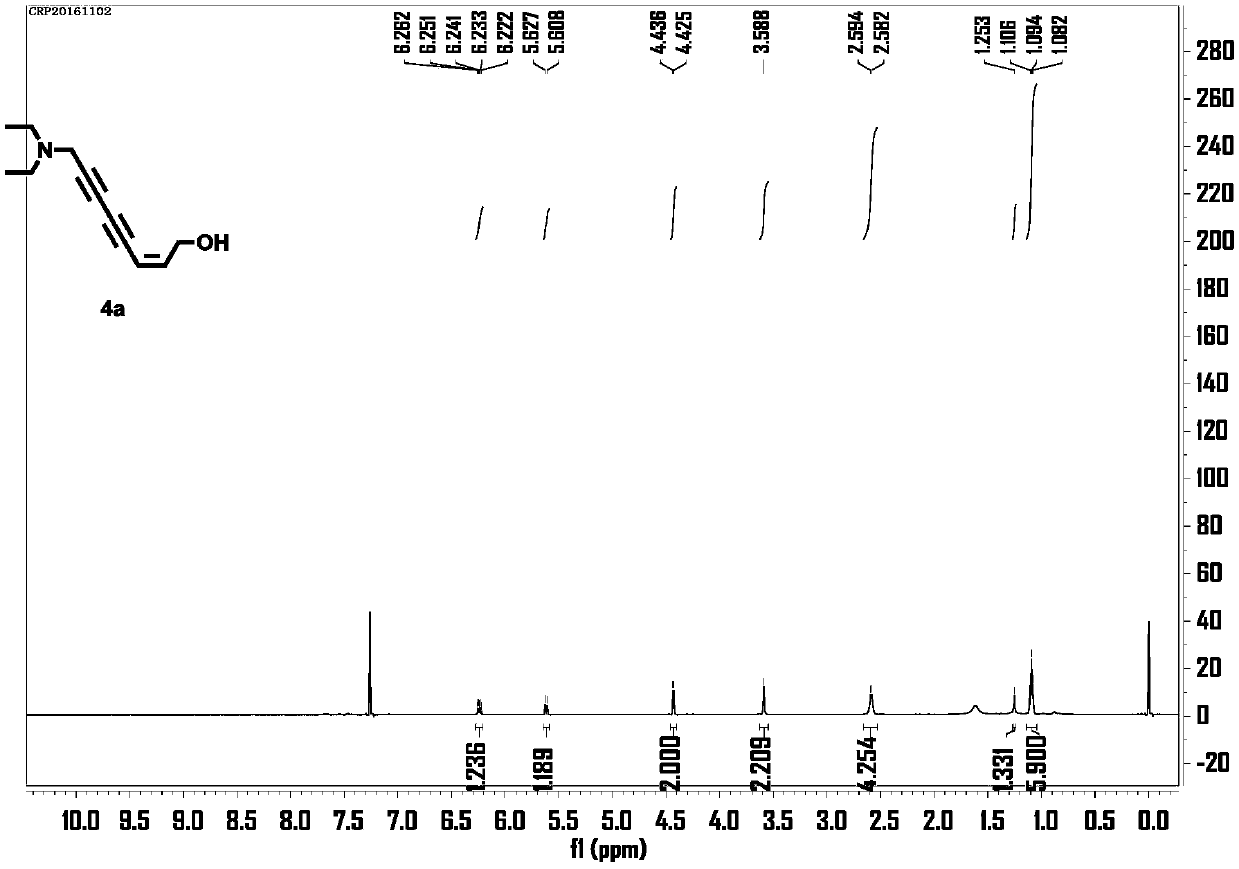
[0041] This experiment proves that the compound (Z)-8-(diethylamino)-2-octene-4,6-diyn-1-ol has a protective effect on PC12 cells damaged by corticosterone, and the specific operations are as follows:
[0042] Cell viability was determined by MTT assay. PC12 cells (2×104 per well) were inoculated in 96-well culture plates, respectively coated with polylysine (0.01%), and after culturing for 24 h, PC12 cells were treated with corticosterone (400 μmol L -1 ) and corticosterone (400μmol·L -1 ) plus different concentrations of (Z)-8-(diethylamino)-2-octene-4,6-diyn-1-ol (0.1,1,10,20,50,100μmolL -1 ), incubated after 24h, 10μL 3-(4,5-dimethylthiazole-2)(4,dimethylthiazole-2)2,5-diphenyltetrazolium bromide (5mg·mL -1 ) was added to each well, and after incubation at 37° C. for 4 hours, the culture solution was taken out, and the absorbance was measured with a microplate reader at a wavelength of 570 nm. Cell viabilit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com