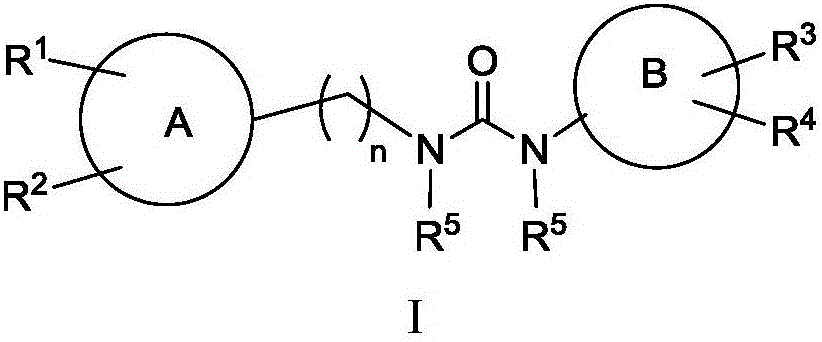
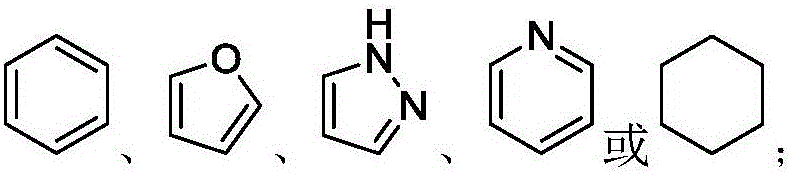
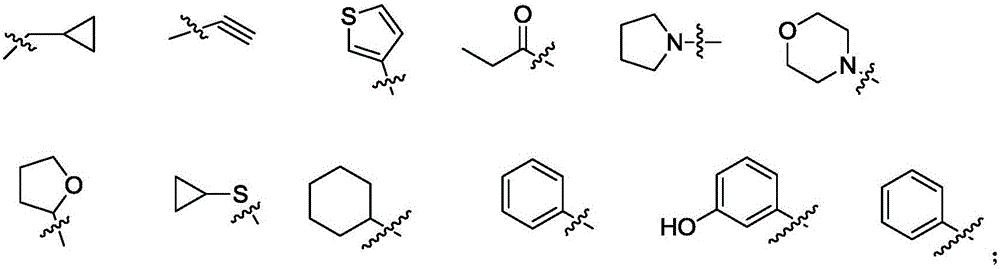
IDH2 (isocitrate dehydrogenase 2) mutant inhibitor and application thereof
A technology of solvents and compounds, applied in the field of medicine, can solve problems such as cell metabolism disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Compounds against IDH 2 / R140Q inhibitory activity assay
[0071] IDH 2 The / R140Q mutant can catalyze the conversion of α-KG to 2-HG and simultaneously oxidize NADPH to NADP+ . Therefore, the inhibitory activity of the compound on the IDH2 / R140Q mutant can be determined by detecting the consumption value of NADPH.
[0072] The detailed method is: add the reaction solution containing 25mM Tris (pH7.4), 150mM NaCl, 10mM MgCl2, 0.03% BSA in a 96-well plate, add 100ng / mL IDH 2 / R140Q and different concentrations of compounds were incubated at 30°C for 1 hour, then 1 mMα-KG and 10 μM NADPH were added, the total system was 200 μL, and the absorbance value of NADPH was continuously detected within 16 hours using a Thermo microplate reader at 30°C and 340 nM wavelength. The final concentration gradient of the compound was set to (10000, 5000, 1000, 500, 100, 50, 10, 5, 1, 0.5, 0.1, 0.01) nM, and the inhibitory activity IC of the compound on IDH2 / R140Q was calcula...
Embodiment 2
[0073] Example 2: Compounds against IDH 2 / R172K inhibitory activity assay
[0074] IDH2 / R172K mutant can catalyze the conversion of α-KG to 2-HG and simultaneously oxidize NADPH to NADP + . Therefore, the inhibitory activity of the compound on the IDH2 / R172K mutant can be determined by detecting the consumption value of NADPH.
[0075] Detailed measurement method with reference to embodiment 1.
Embodiment 3
[0076] Example 3: Compounds against wild-type IDH 2 Inhibitory Activity Determination of
[0077] Wild-type IDH2 in NADP + Under the auxiliary effect, the isocitrate ICT is catalyzed into α-KG, accompanied by the generation of NADPH. Therefore, the inhibitory activity of the compound on IDH2 can be determined by detecting the increase of NADPH.
[0078] The detailed method is as follows: add a reaction solution containing 50mM Tris (pH7.4), 5mM MgCl2, 0.03% BSA to a 96-well plate, add 100ng / mL IDH2 and compounds of different concentrations, incubate at 30°C for 1 hour, and then add 200μM ICT and 200 μM NADP + , the total system was 200 μL, and the change in the absorbance value of NADPH within 16 hours was continuously detected at 30° C. and 340 nM wavelength using a Thermo microplate reader. The final concentration gradient of the compound was set to (10000, 5000, 1000, 500, 100, 50, 10, 5, 1, 0.5, 0.1, 0.01) nM, and the inhibitory activity IC of the compound on IDH2 was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com