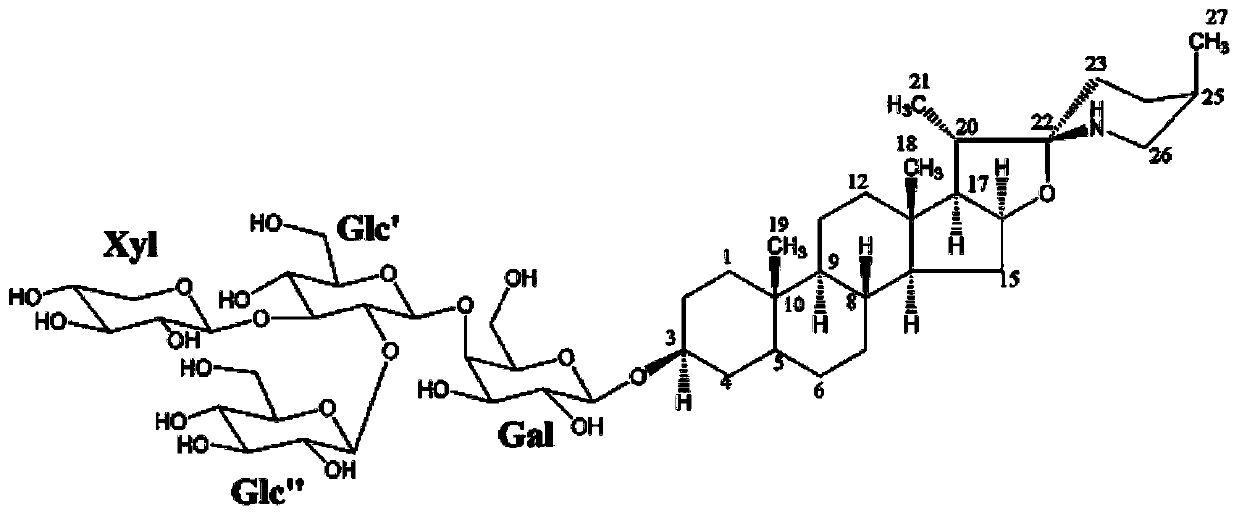
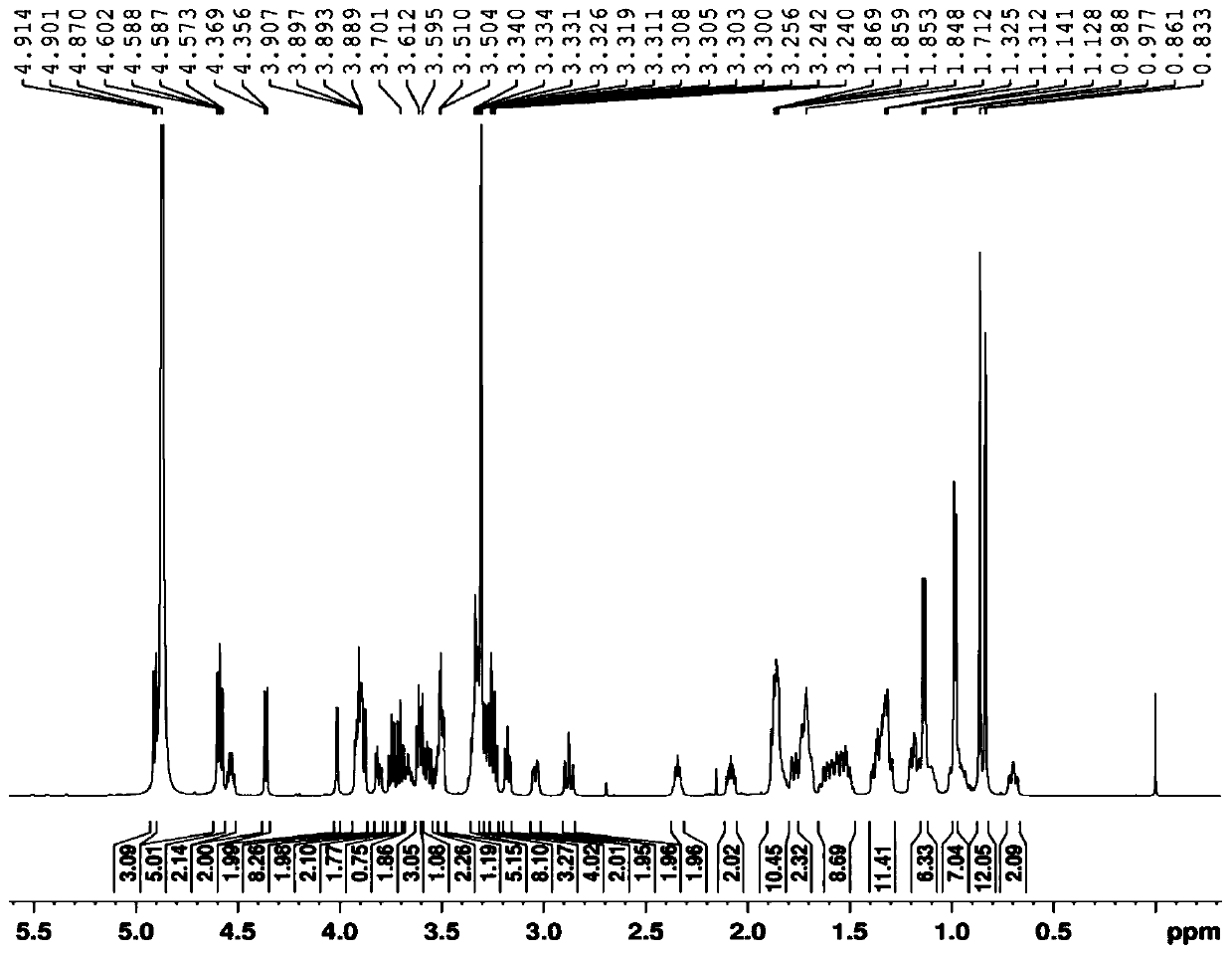
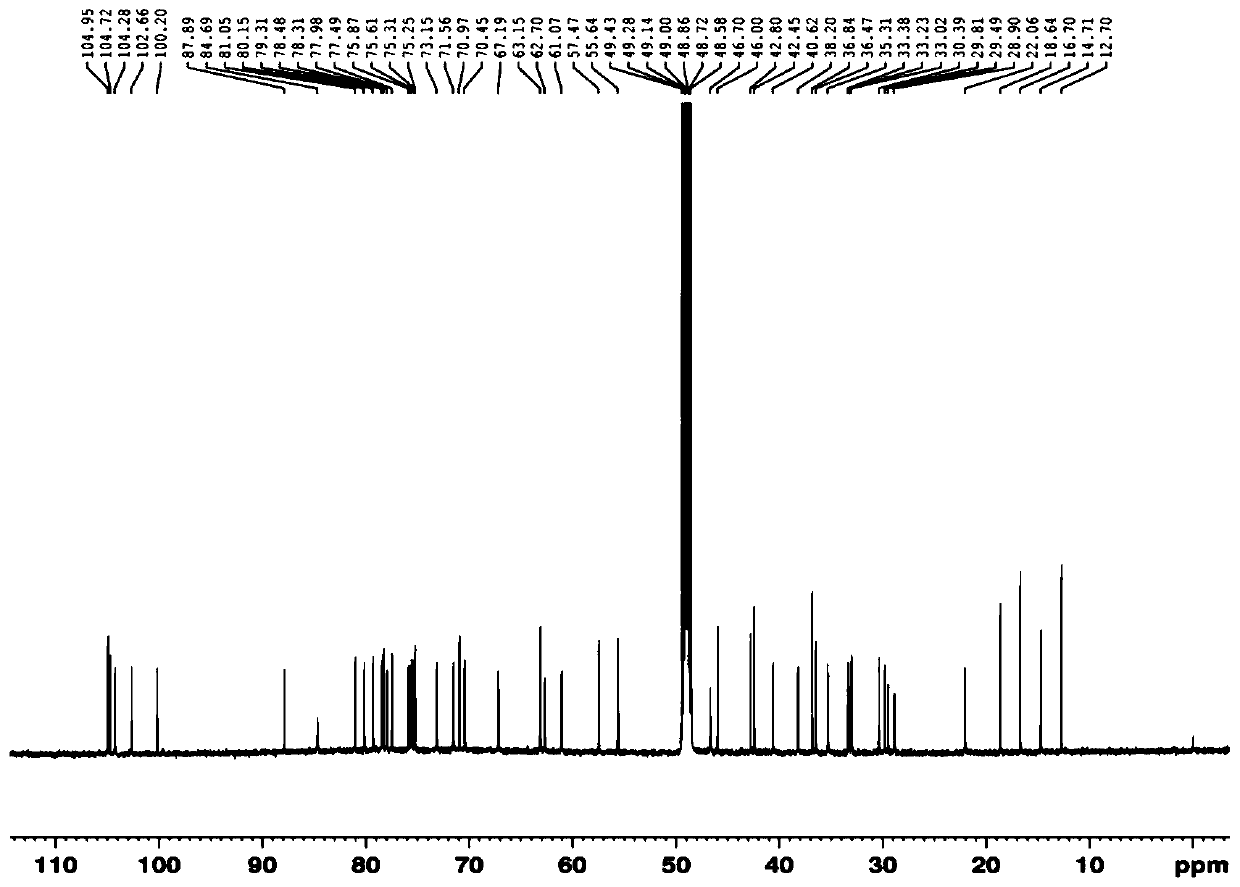
Novel use of a compound and its preparation method
A technology of compounds and uses, applied in the field of medicinal chemistry, to achieve the effect of inhibiting lung cancer cells, inhibiting cancer cells, and improving the purity of separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation method of the compound represented by formula (I) described in this embodiment includes the following steps:
[0047]
[0048] (1) Take 10kg of dried whole plant of Baiying, put it in a reflux bottle, add 80L of ethanol aqueous solution with a volume concentration of 70%, reflux and extract 3 times at 100℃, extract 2 hours each time, and carry out the obtained alcohol extract Filter, combine the resulting filtrate and concentrate it under reduced pressure to an extract with a relative density of 1.05 (measured at 50°C). Add 10 times the mass of the extract to the extract for dispersion, filter, and add the resulting filtrate to D151 Ion exchange macroporous adsorption resin column, and then eluted by 3 times the column volume of distilled water, 3 times the column volume of 95% ethanol aqueous solution, discarded the eluate, and then passed 4 times the column volume containing volume The hydrochloric acid with a concentration of 6‰ is eluted with a 95% etha...
Embodiment 2
[0060] The preparation method of the compound represented by formula (I) described in this embodiment includes the following steps:
[0061] (1) Take 10kg of dried whole plant of Baiying, put it in a reflux bottle, add 60L of ethanol solution with a volume concentration of 65%, reflux and extract twice at 90℃, extract 4 hours each time, and extract the obtained alcohol extract Filter, combine the resulting filtrate and concentrate it under reduced pressure to an extract with a relative density of 1.05 (measured at 50°C). Add 8 times the mass of the extract to the extract for dispersion, filter, and add the resulting filtrate to the extract. D151 ion exchange macroporous adsorption resin column, and then eluted with 2 column volumes of distilled water, 4 column volumes of ethanol solution with a volume concentration of 93%, discarded the eluent, and then passed 3 column volumes containing The volume concentration of hydrochloric acid is 7‰, and the volume concentration of hydrochl...
Embodiment 3
[0067] The preparation method of the compound represented by formula (I) described in this embodiment includes the following steps:
[0068] (1) Take 10kg of dried whole plant of Baiying and place it in a reflux bottle, add 100L of ethanol solution with a volume concentration of 75%, reflux and extract 3 times at 110°C, extract 2 hours each time, and combine the obtained alcohol extract Filter, combine the obtained filtrate and concentrate it under reduced pressure to an extract with a relative density of 1.05 (measured at 50°C). Add 12 times the mass of the extract to the extract for dispersion, filter, and add the obtained filtrate to the extract. D151 ion exchange macroporous adsorption resin column, and then eluted by 4 times the column volume of distilled water, 2 times the column volume of the ethanol solution with a volume concentration of 97%, discarded the eluate, and then passed 5 times the column volume containing The volume concentration of hydrochloric acid is 5‰, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com