Application of tanshinone compounds to preparation of antitumor medicines
A technology of anti-tumor drugs and compounds, applied in the application field of tanshinone compounds in the preparation of anti-tumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
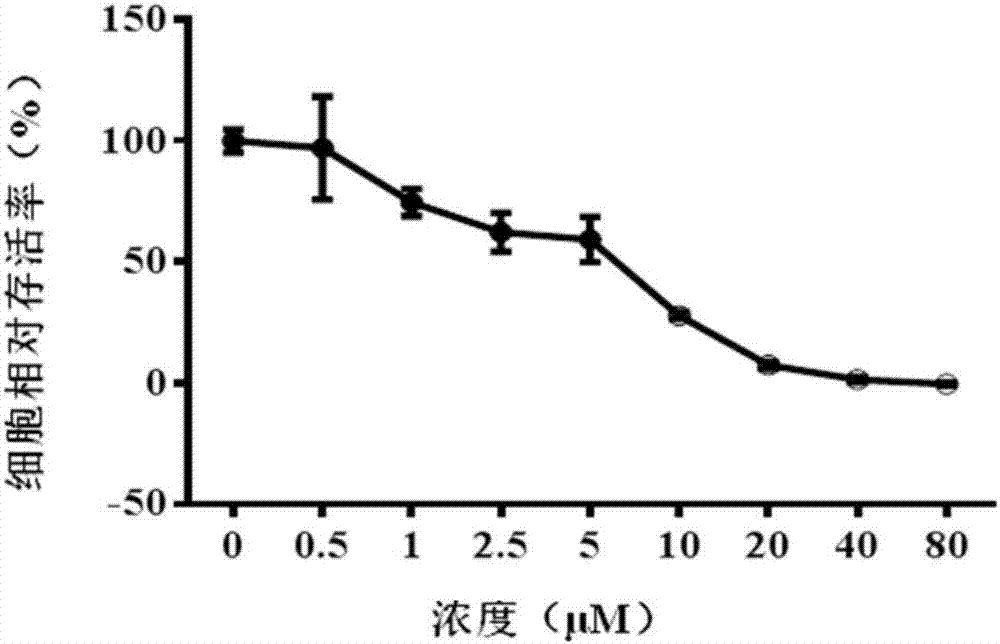
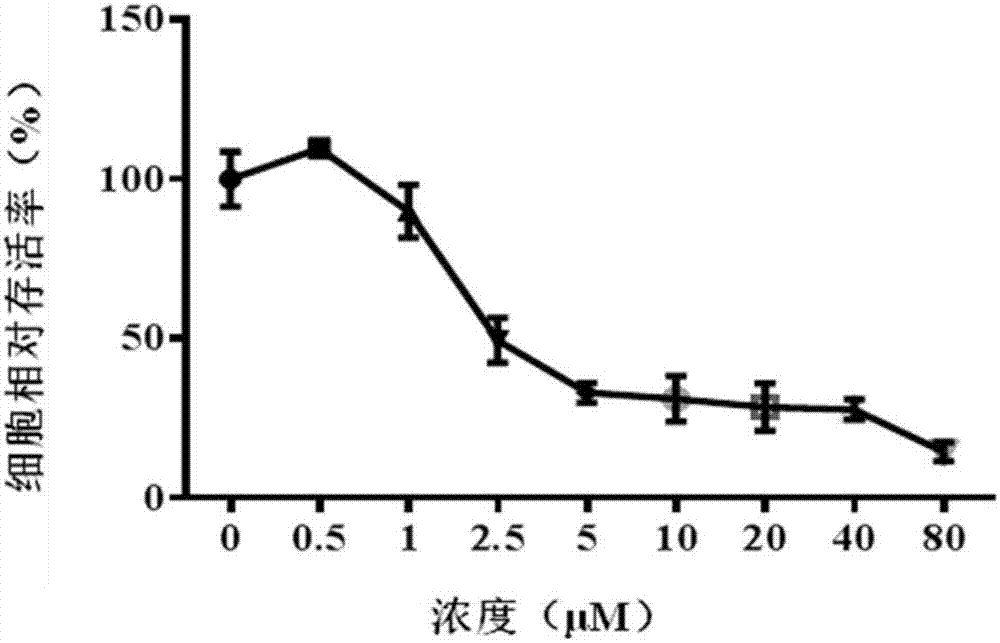
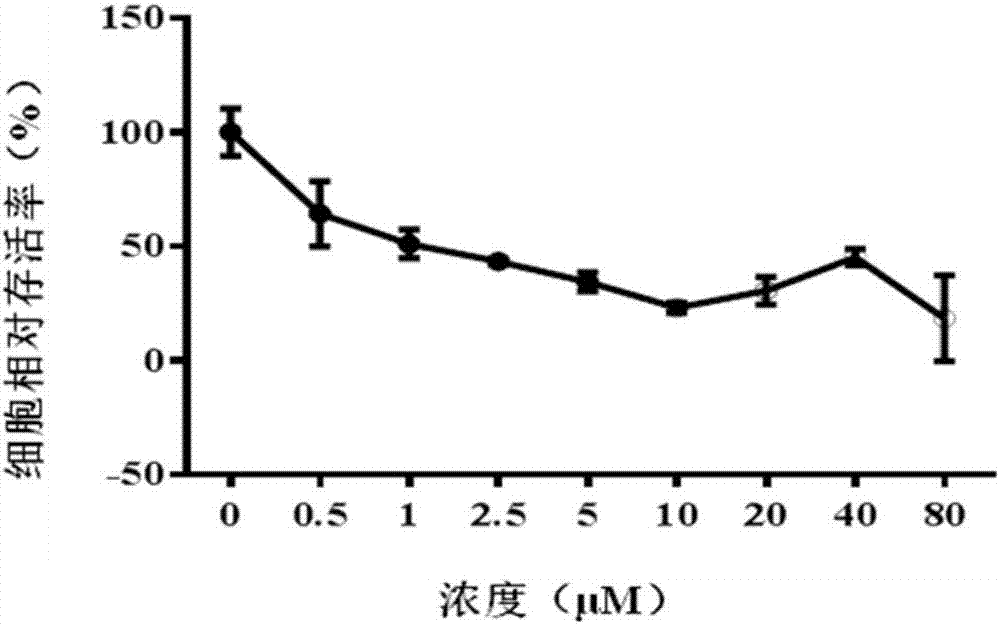
[0032] The inhibitory effect of CCK-8 method Tanshinone compounds on tumor cell proliferation
[0033] The four tanshinone compounds prepared in Example 1.1 were prepared into different concentrations of drug-containing solutions with serum-free medium. Human lung cancer cell A549 and pancreatic cancer cell DT6606 were used as experimental subjects. The logarithmic growth phase was in good condition. The A549 cells and DT6606 cells were seeded in a 96-well plate with a seeding concentration of 5000 cells / well and placed in a conventional cell incubator for 12 hours. The tumor cells were processed according to the previous dosing schedule, and the drug-free medium was used as a negative control. After 24 hours of culturing, the CCK-8 method was used to determine the cell viability after drug action, and the in vitro anti-tumor activity of the four tanshinone compounds was analyzed. The specific steps are:
[0034] a) Administration: As the four tanshinone compounds choose to use 1...
experiment example 2
[0045] Annexin V / PI double staining method to evaluate the apoptosis-inducing effect of four tanshinone compounds on lung cancer cell line A549
[0046] Take A549 cells in logarithmic growth phase and adjust the cell density to 2×10 4 Pieces / well, inoculated in a 24-well plate, 1 mL per well, after normal incubation for 12 hours, discard the medium, and add different concentrations (1 μM, 5 μM and 10 μM) of the four tanshinone compounds prepared in Example 1.1 for 24 hours. And set up a normal control group, with three replicate holes in each group.
[0047] Discard the supernatant in the culture plate, wash the cells twice with PBS, add trypsin without EDTA for 1 min, stop with serum-containing medium, collect the cells in the same centrifuge tube, centrifuge at 1000 rpm for 3 min, discard the supernatant .
[0048] After adding PBS, centrifuge at 1000 rpm for 3 min, and discard the supernatant. Wash twice, add the pre-prepared 1×AnnexinV Binding Solution to make the final concent...
Embodiment 1
[0054] Method for preparing and chemical structure identification of said tanshinone compounds
[0055] 1.1 Extraction and separation
[0056] The crude extract of the ethyl acetate part of Salvia miltiorrhiza was 2.5 kg, and enough dichloromethane was added to dissolve it completely, and then transferred to a separatory funnel. Add 2 times the volume of water to the separatory funnel and mix gently, stand still and separate to obtain the dichloromethane layer, which is transferred to a round bottom flask and concentrated to the state of extract using a rotary evaporator to obtain ethyl salvia miltiorrhiza. Site extract.
[0057] Dissolve the extract of the ethyl acetate part of Salvia miltiorrhiza with an appropriate amount of ethyl acetate, mix it with 4.9 kg of sample-mixing silica gel (200-300 mesh) and grind it. After the solvent has evaporated, pass through a 60-mesh sieve and load the sample. Use different ratios of petroleum ether: ethyl acetate (20:1, 10:1, 5:1, 1:1) to pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com