Whole-course tracing safety management system based on minimum packing unit of vaccines
A safety management and vaccine technology, applied in the field of full traceability safety management system, can solve the problems of difficulty in unified management of vaccines, lack of a comprehensive and timely emergency management mechanism, and suspension of use, so as to remove monitoring blind spots, realize intelligence, and improve real-time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The technical solutions of the present invention will be further described in detail below in conjunction with specific embodiments.
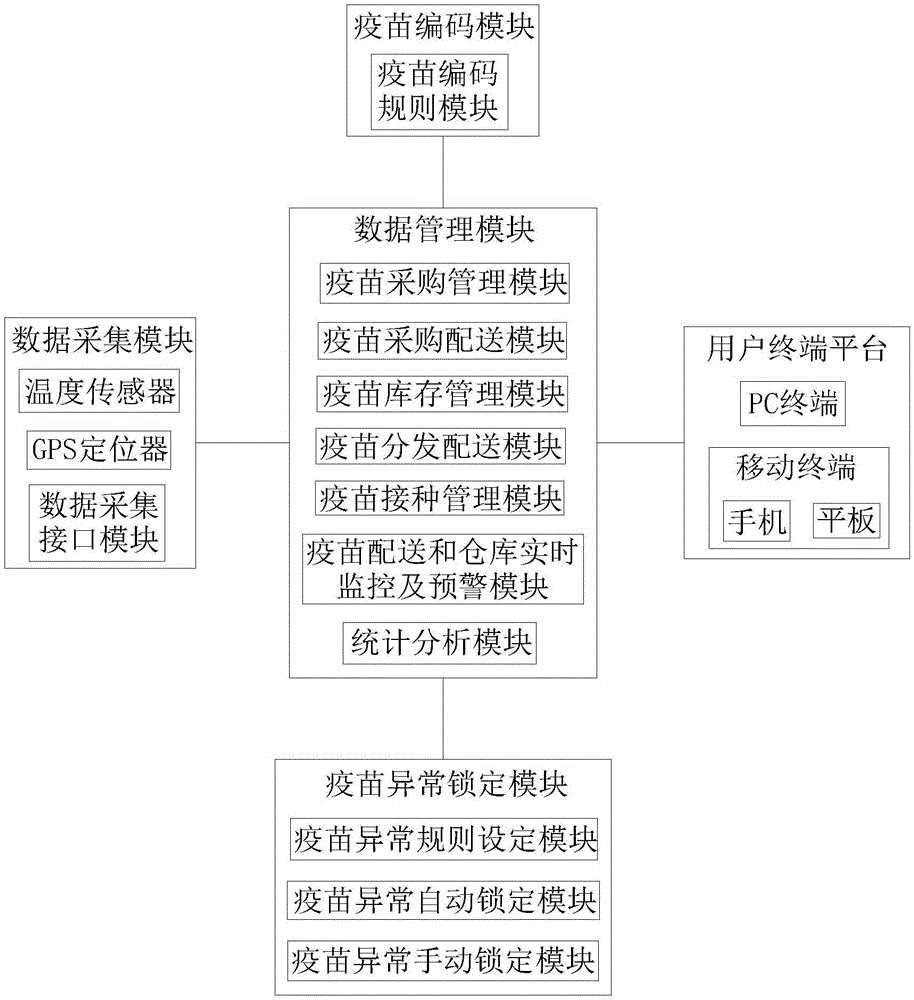
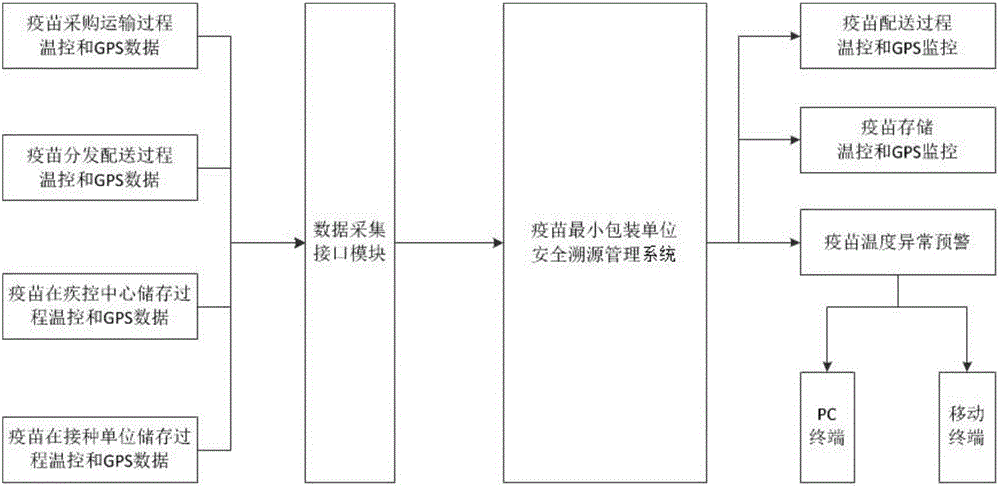
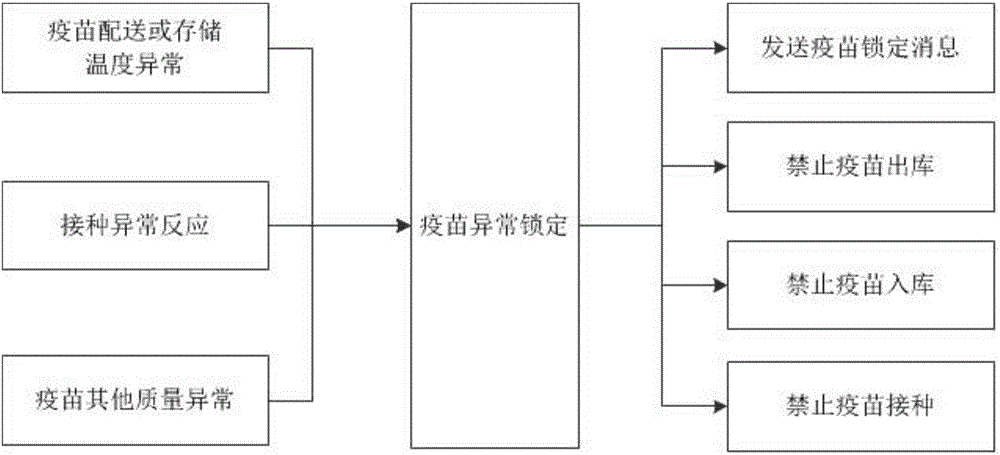
[0031] see Figure 1-3 , a full-process traceability safety management system based on the smallest packaging unit of vaccines, including a vaccine coding module, a data collection module, a data management module, a vaccine abnormal locking module, and a user terminal platform; the vaccine coding module is connected to the data management module, and the vaccine The connection mode between the coding module and the data management module is not limited. In this embodiment, preferably, the vaccine coding module is connected to the data management module through a local area network or a wireless network; the data collection module is connected to the data management module. Connection, the connection mode between the data acquisition module and the data management module is not limited, in the present embodiment, preferably, the data acq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com